| |

| |
| Names | |
|---|---|
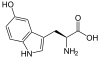
| IUPAC name
2-amino-3-(5-hydroxy-1H-indol-3-yl)propanoic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.022.193 |
| KEGG | |
| MeSH | 5-Hydroxytryptophan |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| Properties | |
| C11H12N2O3 | |
| Molar mass | 220.228 g·mol−1 |
| Density | 1.484 g/mL |
| Melting point | 298 to 300 °C (568 to 572 °F; 571 to 573 K) |
| Boiling point | 520.6 °C (969.1 °F; 793.8 K) |
| Pharmacology | |
| N06AX01 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
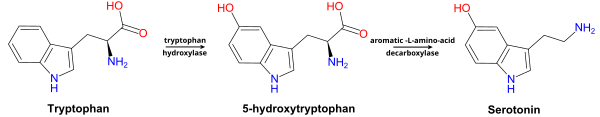
5-Hydroxytryptophan (5-HTP), also known as oxitriptan, is a naturally occurring amino acid and chemical precursor as well as a metabolic intermediate in the biosynthesis of the neurotransmitter serotonin.
Uses
5-HTP is sold over the counter in the United States, Canada, the Netherlands, and the United Kingdom as a dietary supplement for use as an antidepressant, appetite suppressant, and sleep aid. It is also marketed in many European countries for the indication of major depression under the trade names Cincofarm, Levothym, Levotonine, Oxyfan, Telesol, Tript-OH, and Triptum.
A 2002 review concluded that although the data evaluated suggests
that 5-HTP is more effective than placebo in the treatment of
depression, the evidence was insufficient to be conclusive due to a lack
of clinical data meeting the rigorous standards of today. More and larger studies using current methodologies are needed to determine if 5-HTP is truly effective in treating depression.
In small controlled trials 5-HTP has also been reported to augment the
antidepressant efficacy of the antidepressant clomipramine.
5-HTP is sometimes taken by people coming down from MDMA to relieve post-MDMA dysphoria. As 5-HTP is a necessary precursor for the brain to produce more serotonin,
and MDMA use depletes a person's natural serotonin levels, it is
believed that taking 5-HTP after consuming MDMA will speed up serotonin
production. DanceSafe claims that the anecdotal evidence is widespread and that the theory is physiologically reasonable.
Backing up this approach is research conducted by Wang, et al. in 2007,
which observed that MDMA-induced depletions of 5-HT (serotonin) were
restored in rats after administration of 5-HTP, and suggested that this
approach might be clinically useful in abstinent MDMA users.
At high doses, or in combination with carbidopa, it has been used off-label to treat obesity (by promoting weight loss).
In clinical trials of various design, 5-HTP has also been reported to treat fibromyalgia, myoclonus, migraine, and cerebellar ataxia.
However, these clinical findings, as for all therapeutic findings with
5-HTP, are preliminary and need confirmation in larger trials.
5-HTP's drawbacks as a drug
The short half-life (<2h 5-htp="" adverse="" are="" as="" associated="" burden="" c="" dosing.="" even="" event="" exposure="" fluctuate="" fluctuations="" frequent="" from="" increased="" inherently="" levels="" limit="" may="" of="" potential="" relatively="" resulting="" sub="" substantially="" such="" systemic="" the="" therapeutic="" usually="" will="" with="">max
drug spikes, and decreased clinical efficacy resulting from
sub-therapeutic exposure for large parts of the day. It has been
proposed that 5-HTP dosage forms achieving prolonged delivery would be
more effective, as is generally the situation with short-acting active pharmaceutical ingredients.Side Effects
Potential side effects of 5-HTP include heartburn, stomach pain, nausea, vomiting, diarrhea, drowsiness, sexual problems, vivid dreams or nightmares, and muscle problems.
Because 5-HTP has not been thoroughly studied in a clinical setting,
possible side effects and interactions with other drugs are not well
known. According to the US National Institute of Health TOXNET, 5-HTP
has not been associated with serotonin syndrome or any serious adverse
events in humans. Across multiple studies, 5-HTP has also been reported to not cause any noticeable hematological or cardiovascular changes. 5-HTP has also been associated with eosinophilia, but later studies have not found any causal connection.
Interactions
When combined with antidepressants of the MAOI or SSRI class, very high parenteral doses of 5-HTP can cause acute serotonin syndrome in rats.
It is unclear if such findings have clinical relevance, as most drugs
will cause serious adverse events or death in rodents at very high
doses. In humans 5-HTP has never been clinically associated with
serotonin syndrome, although a case report suggests 5-HTP can
precipitate mania when added to an MAOI.
When combined with carbidopa (as a treatment for symptoms of Parkinson's disease), 5-HTP causes nausea and vomiting; however this can be alleviated via administration of granisetron. As mentioned below under pharmacology, cases of scleroderma-like illness have been reported in patients using carbidopa and 5-HTP.
Oral 5-HTP results in an increase in urinary 5-HIAA,
a serotonin metabolite, indicating that 5-HTP is peripherally
metabolized to serotonin, which is then metabolized. This might cause a
false positive test in tests looking for carcinoid syndrome.
Due to the conversion of 5-HTP into serotonin by the liver, there could
be a risk of heart valve disease from serotonin's effect on the heart,
as based on preclinical findings. However, 5-HTP has not been associated with cardiac toxicity in humans.
It has been suggested that 5-HTP may cause eosinophilia-myalgia syndrome
(EMS), a serious condition which results in extreme muscle tenderness,
myalgia, and blood abnormalities. However, there is evidence to show
that EMS was likely caused by a contaminant in certain 5-HTP
supplements.
Production
5-HTP is produced from the amino acid tryptophan through the action of the enzyme tryptophan hydroxylase. Tryptophan hydroxylase is one of the biopterin-dependent aromatic amino acid hydroxylases.
Production of 5-HTP is the rate-limiting step in 5-HT synthesis. 5-HTP
is normally rapidly converted to 5-HT by amino acid decarboxylase.
Absorption
After oral administration, 5-HTP is absorbed by the upper intestine.
The mode of absorption is not known, but presumably involves active
transport via amino acid transporters. The oral bioavailability of 5-HTP
has not been determined. With a decarboxylase inhibitor, the
bioavailability of 5-HTP can be higher than 50%.
Pharmacokinetics
5-HTP
is rapidly absorbed with Tmax of ~1.5h, and rapidly eliminated with a
half-life of ~1.5-2h. Co-administration of a decarboxylase inhibitor
(e.g. carbidopa, benserazide) doubles the half-life of 5-HTP, to 3-4h, and enhances exposure several fold, depending on the dosing regimen.
Metabolism
5-HTP is decarboxylated to serotonin (5-hydroxytryptamine or 5-HT) by the enzyme aromatic-L-amino-acid decarboxylase with the help of vitamin B6. This reaction occurs both in nervous tissue and in the liver. 5-HTP crosses the blood–brain barrier, while 5-HT does not. Excess 5-HTP, especially when administered with vitamin B6, is thought to be metabolized and excreted.
| 5-HTP | AAAD | Serotonin | |
|

| ||
| PLP | |||

| |||
Pharmacology
The psychoactive action of 5-HTP is derived from its increase in production of serotonin in central nervous system tissue.
Research shows that co-administration with carbidopa greatly increases plasma 5-HTP levels.
Other studies have indicated the risk of a scleroderma-like condition resulting from the combination of 5-HTP and carbidopa.
Regulatory Status
There are currently no approved drug products containing 5-HTP approved by the FDA. All available 5-HTP products are nutraceuticals
and are as such not regulated or verified for purity, integrity, or
clinical efficacy or safety, mandating caution regarding human
consumption.
5-HTP Slow-Release
5-HTP's
fast-in, fast-out pharmacokinetics is impractical for chronic drug
therapy. Research conducted at Duke University in mice have demonstrated
that 5-HTP when administered as slow-release appears to gain drug
properties. Slow-Release delivery attenuates or abolishes the peaks and valleys in 5-HTP exposure during treatment.
Slow-release delivery of 5-HTP markedly improved the safety profile of
5-HTP and conferred stable plasma exposure of 5-HTP and strong and
sustained enhancement of brain serotonin function.
This discovery indicates that 5-HTP slow-release medications represent a
new avenue for treatment of brain disorders responsive to serotonergic
enhancement.
Dietary sources
Though 5-HTP is found in food only in insignificant quantities, it is
a chemical involved intermediately in the metabolism of tryptophan, an
amino acid found in milk, meat, potatoes, pumpkin, and various greens.
The seeds of the Griffonia simplicifolia, a climbing shrub native to West Africa and Central Africa, are used as an herbal supplement for their 5-hydroxytryptophan (5-HTP) content. In one 2010 trial, Griffonia simplicifolia extract appeared to increase satiety in overweight women.