| |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌmɛləˈtoʊnɪn/ |
| Trade names | Many |
| Other names | N-acetyl-5-methoxy tryptamine |
| AHFS/Drugs.com | Consumer Drug Information |
| License data | |
| Routes of administration | By mouth, sublingual, transdermal |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 30–50% |
| Metabolism | Liver via CYP1A2 mediated 6-hydroxylation |
| Metabolites | 6-hydroxymelatonin, N-acetyl-5lhydroxytryptamine, 5-methoxytryptamine |
| Elimination half-life | 30–50 minutes |
| Excretion | Kidney |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.725 |
| Chemical and physical data | |
| Formula | C13H16N2O2 |
| Molar mass | 232.283 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 117 °C (243 °F) |
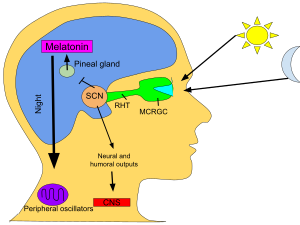
Melatonin is a hormone that regulates the sleep–wake cycle. It is primarily released by the pineal gland. As a supplement, it is often used for the short-term treatment of trouble sleeping such as from jet lag or shift work. Evidence of benefit, however, is unclear. One review found onset of sleep occurred 6 minutes faster with use but found no change in total time asleep. It may work as well as the medication ramelteon. It is typically taken by mouth.
Side effects from supplements are minimal at low doses for short durations. Side effects may include sleepiness, headaches, nausea, diarrhea, and abnormal dreams. Use is not recommended during pregnancy or breastfeeding. Use is also not recommended in those with liver problems.
In animals (including humans), melatonin is involved in synchronizing the circadian rhythm including sleep–wake timing, blood pressure regulation, and seasonal reproduction. Many of its effects are through activation of the melatonin receptors, while others are due to its role as an antioxidant. In plants it functions to defend against oxidative stress. Melatonin is also present in various foods.
Melatonin was discovered in 1958. It is sold over the counter in Canada and the United States. In the United Kingdom it is a prescription-only medication. A month's supply costs about US $1 to 4 in the United States. In the United Kingdom a month's supply costs the NHS about 15 pounds. It is not FDA-approved for any use. In Australia and Europe, it is approved for trouble sleeping in people over the age of 54.
Medical use
Sleep disorders
Positions on the benefits of melatonin for insomnia are mixed. An Agency for Healthcare Research and Quality (AHRQ) review from 2015 stated that evidence of benefit in the general population was unclear. A review from 2017 found a modest effect on time until onset of sleep. Another review from 2017 put this decrease at 6 minutes to sleep onset but found no difference in total sleep time. Melatonin may also be useful in delayed sleep phase syndrome. Melatonin appears to work as well as ramelteon but costs less.
Melatonin is a safer alternative than clonazepam in the treatment of REM sleep behavior disorder – a condition associated with the synucleinopathies like Parkinson's disease and dementia with Lewy bodies. In Europe it is used for short-term treatment of insomnia in people who are 55 years old or older. It is deemed to be a first line agent in this group.
Melatonin reduces the time until onset of sleep and increases sleep duration in children with neurodevelopmental disorders.
Dementia
A 2016 Cochrane review found no evidence that melatonin helped sleep problems in people with moderate to severe dementia due to Alzheimer's disease.
A 2019 review found that while melatonin may improve sleep in minimal
cognitive impairment, after the onset of Alzheimer's it has little to no
effect. Melatonin may, however, help with sundowning.
Jet lag and shift work
Melatonin is known to reduce jet lag, especially in eastward travel. If the time it is taken is not correct, however, it can instead delay adaption.
Melatonin appears to have limited use against the sleep problems of people who work shift work. Tentative evidence suggests that it increases the length of time people are able to sleep.
Adverse effects
Melatonin appears to cause very few side effects as tested in the short term, up to three months, at low doses.
Two systematic reviews found no adverse effects of exogenous melatonin
in several clinical trials and comparative trials found the adverse
effects headaches, dizziness, nausea, and drowsiness were reported about
equally for both melatonin and placebo. Prolonged-release melatonin is safe with long-term use of up to 12 months.
Although not recommended for long term use beyond this, low-dose
melatonin is generally safer, and a better alternative, than many
prescription and over the counter sleep aids if a sleeping medication
must be used for an extended period of time. Low-doses of melatonin are
usually sufficient to produce a hypnotic
effect in most people. Higher doses do not appear to result in a
stronger effect, but instead appear to cause drowsiness for a longer
period of time.
Melatonin can cause nausea, next-day grogginess, and irritability. In the elderly, it can cause reduced blood flow and hypothermia. In autoimmune disorders, evidence is conflicting whether melatonin supplementation may ameliorate or exacerbate symptoms due to immunomodulation.
Melatonin can lower follicle-stimulating hormone levels. Melatonin's effects on human reproduction remain unclear.
In those taking warfarin, some evidence suggests there may exist a potentiating drug interaction, increasing the anticoagulant effect of warfarin and the risk of bleeding.
Functions
When
eyes receive light from the sun, the pineal gland's production of
melatonin is inhibited and the hormones produced keep the human awake.
When the eyes do not receive light, melatonin is produced in the pineal
gland and the human becomes tired.
Circadian rhythm
In animals, melatonin plays an important role in the regulation of sleep–wake cycles.
Human infants' melatonin levels become regular in about the third month
after birth, with the highest levels measured between midnight and 8:00
am. Human melatonin production decreases as a person ages. Also, as children become teenagers, the nightly schedule of melatonin release is delayed, leading to later sleeping and waking times.
Antioxidant
Melatonin was first reported as a potent antioxidant and free radical scavenger in 1993. In vitro, melatonin acts as a direct scavenger of oxygen radicals and reactive nitrogen species including OH•, O2−•, and NO•. In plants, melatonin works with other antioxidants to improve the overall effectiveness of each antioxidant. Melatonin has been proven to be twice as active as vitamin E, believed to be the most effective lipophilic antioxidant. Via signal transduction through melatonin receptors, melatonin promotes the expression of antioxidant enzymes such as superoxide dismutase, glutathione peroxidase, glutathione reductase, and catalase.
Melatonin occurs at high concentrations within mitochondrial fluid which greatly exceed the plasma concentration of melatonin.
Due to its capacity for free radical scavenging, indirect effects on
the expression of antioxidant enzymes, and its significant
concentrations within mitochondria, a number of authors have indicated
that melatonin has an important physiological function as a
mitochondrial antioxidant.
The melatonin metabolites produced via the reaction of melatonin with reactive oxygen species or reactive nitrogen species also react with and reduce free radicals. Melatonin metabolites generated from redox reactions include cyclic 3-hydroxymelatonin, N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK), and N1-acetyl-5-methoxykynuramine (AMK).
Immune system
While it is known that melatonin interacts with the immune system, the details of those interactions are unclear. An antiinflammatory
effect seems to be the most relevant. There have been few trials
designed to judge the effectiveness of melatonin in disease treatment.
Most existing data are based on small, incomplete trials. Any positive
immunological effect is thought to be the result of melatonin acting on
high-affinity receptors (MT1 and MT2) expressed in immunocompetent
cells. In preclinical studies, melatonin may enhance cytokine production, and by doing this, counteract acquired immunodeficiences. Some studies also suggest that melatonin might be useful fighting infectious disease including viral, such as HIV, and bacterial infections, and potentially in the treatment of cancer.
Biosynthesis
Overview of melatonin biosynthesis
In animals, biosynthesis of melatonin occurs through hydroxylation, decarboxylation, acetylation and a methylation starting with L-tryptophan. L-tryptophan is produced in the shikimate pathway from chorismate or is acquired from protein catabolism. First L-tryptophan is hydroxylated on the indole ring by tryptophan hydroxylase to produce 5-hydroxytryptophan. This intermediate (5-HTP) is decarboxylated by pyridoxal phosphate and 5-hydroxytryptophan decarboxylase to produce serotonin. Serotonin is itself an important neurotransmitter, but is also converted into N-acetylserotonin by serotonin N-acetyltransferase with acetyl-CoA. Hydroxyindole O-methyltransferase and S-adenosyl methionine convert N-acetylserotonin into melatonin through methylation of the hydroxyl group.
In bacteria, protists, fungi, and plants, melatonin is
synthesized indirectly with tryptophan as an intermediate product of the
shikimate pathway. In these cells, synthesis starts with D-erythrose 4-phosphate and phosphoenolpyruvate, and in photosynthetic cells with carbon dioxide. The rest of the synthesising reactions are similar, but with slight variations in the last two enzymes.
It has been hypothesized that melatonin is made in the mitochondria and chloroplasts.
Mechanism
Mechanism of melatonin biosynthesis
In order to hydroxylate L-tryptophan, the cofactor tetrahydrobiopterin
(THB) must first react with oxygen and the active site iron of
tryptophan hydroxylase. This mechanism is not well understood, but two
mechanisms have been proposed:
1. A slow transfer of one electron from the THB to O2 could produce a superoxide which could recombine with the THB radical
to give 4a-peroxypterin. 4a-peroxypterin could then react with the
active site iron (II) to form an iron-peroxypterin intermediate or
directly transfer an oxygen atom to the iron.
2. O2 could react with the active site iron (II)
first, producing iron (III) superoxide which could then react with the
THB to form an iron-peroxypterin intermediate.
Iron (IV) oxide from the iron-peroxypterin intermediate is selectively attacked by a double bond to give a carbocation
at the C5 position of the indole ring. A 1,2-shift of the hydrogen and
then a loss of one of the two hydrogen atoms on C5 reestablishes aromaticity to furnish 5-hydroxy-L-tryptophan.
A decarboxylase with cofactor pyridoxal phosphate (PLP) removes CO2 from 5-hydroxy-L-tryptophan to produce 5-hydroxytryptamine. PLP forms an imine with the amino acid derivative. The amine on the pyridine is protonated and acts as an electron sink, enabling the breaking of the C-C bond and releasing CO2.
Protonation of the amine from tryptophan restores the aromaticity of
the pyridine ring and then imine is hydrolyzed to produce
5-hydroxytryptamine and PLP.
It has been proposed that histidine residue His122 of serotonin N-acetyl transferase is the catalytic residue that deprotonates
the primary amine of 5-hydroxytryptamine, which allows the lone pair on
the amine to attack acetyl-CoA, forming a tetrahedral intermediate. The
thiol from coenzyme A serves as a good leaving group when attacked by a general base to give N-acetylserotonin.
N-acetylserotonin is methylated at the hydroxyl position by S-adenosyl methionine (SAM) to produce S-adenosyl homocysteine (SAH) and melatonin.
Regulation
In vertebrates, melatonin secretion is regulated by activation of the beta-1 adrenergic receptor by norepinephrine.
Norepinephrine elevates the intracellular cAMP concentration via
beta-adrenergic receptors and activates the cAMP-dependent protein
kinase A (PKA). PKA phosphorylates the penultimate enzyme, the
arylalkylamine N-acetyltransferase (AANAT). On exposure to (day)light,
noradrenergic stimulation stops and the protein is immediately destroyed
by proteasomal proteolysis. Production of melatonin is again started in the evening at the point called the dim-light melatonin onset.
Blue light, principally around 460–480 nm, suppresses melatonin biosynthesis,
proportional to the light intensity and length of exposure. Until
recent history, humans in temperate climates were exposed to few hours
of (blue) daylight in the winter; their fires gave predominantly yellow
light. The incandescent light bulb widely used in the 20th century produced relatively little blue light. Light containing only wavelengths greater than 530 nm does not suppress melatonin in bright-light conditions.
Wearing glasses that block blue light in the hours before bedtime may
decrease melatonin loss. Use of blue-blocking goggles the last hours
before bedtime has also been advised for people who need to adjust to an
earlier bedtime, as melatonin promotes sleepiness.
Pharmacology
Pharmacodynamics
In humans, melatonin is a full agonist of melatonin receptor 1 (picomolar binding affinity) and melatonin receptor 2 (nanomolar binding affinity), both of which belong to the class of G-protein coupled receptors (GPCRs). Melatonin receptors 1 and 2 are both Gi/o-coupled GPCRs, although melatonin receptor 1 is also Gq-coupled. Melatonin also acts as a high-capacity free radical scavenger within mitochondria which also promotes the expression of antioxidant enzymes such as superoxide dismutase, glutathione peroxidase, glutathione reductase, and catalase via signal transduction through melatonin receptors.
Pharmacokinetics
When used several hours before sleep according to the phase response curve for melatonin in humans, small amounts (0.3 mg) of melatonin shift the circadian clock earlier, thus promoting earlier sleep onset and morning awakening.
Melatonin is rapidly absorbed and distributed, reaching peak plasma
concentrations after 60 minutes of administration, and is then
eliminated. Melatonin has a half life of 35–50 minutes.
In humans, 90% of orally administered exogenous melatonin is cleared in
a single passage through the liver, a small amount is excreted in
urine, and a small amount is found in saliva. The bioavalibility of melatonin is between 10 and 50%.
Melatonin is metabolised in the liver by cytochrome P450 enzyme
CYP1A2 to 6-hydroxymelatonin. Metabolites are conjugated with sulfuric
acid or glucuronic acid for excretion in the urine. 5% of melatonin is
excreted in the urine as the unchanged drug.
Some of the metabolites formed via the reaction of melatonin with a free radical include cyclic 3-hydroxymelatonin, N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK), and N1-acetyl-5-methoxykynuramine (AMK).
The membrane transport proteins that move melatonin across a membrane include, but are not limited to, glucose transporters, including GLUT1, and the proton-driven oligopeptide transporters PEPT1 and PEPT2.
History
Melatonin was first discovered in connection to the mechanism by which some amphibians and reptiles change the color of their skin. As early as 1917, Carey Pratt McCord and Floyd P. Allen discovered that feeding extract of the pineal glands of cows lightened tadpole skin by contracting the dark epidermal melanophores.
In 1958, dermatology professor Aaron B. Lerner and colleagues at Yale University, in the hope that a substance from the pineal might be useful in treating skin diseases, isolated the hormone from bovine pineal gland extracts and named it melatonin. In the mid-70s Lynch et al. demonstrated that the production of melatonin exhibits a circadian rhythm in human pineal glands.
The discovery that melatonin is an antioxidant was made in 1993.
The first patent for its use as a low-dose sleep aid was granted to Richard Wurtman at MIT in 1995. Around the same time, the hormone got a lot of press as a possible treatment for many illnesses. The New England Journal of Medicine
editorialized in 2000: "With these recent careful and precise
observations in blind persons, the true potential of melatonin is
becoming evident, and the importance of the timing of treatment is
becoming clear."
Other animals
In vertebrates, melatonin is produced in darkness, thus usually at night, by the pineal gland, a small endocrine gland
located in the center of the brain but outside the blood–brain barrier. Light/dark information reaches the suprachiasmatic nuclei from retinal photosensitive ganglion cells of the eyes
rather than the melatonin signal (as was once postulated). Known as
"the hormone of darkness", the onset of melatonin at dusk promotes
activity in nocturnal (night-active) animals and sleep in diurnal ones including humans.
Many animals use the variation in duration of melatonin production each day as a seasonal clock. In animals including humans,
the profile of melatonin synthesis and secretion is affected by the
variable duration of night in summer as compared to winter. The change
in duration of secretion thus serves as a biological signal for the
organization of daylength-dependent (photoperiodic) seasonal functions such as reproduction, behavior, coat growth, and camouflage coloring in seasonal animals.
In seasonal breeders that do not have long gestation periods and that
mate during longer daylight hours, the melatonin signal controls the
seasonal variation in their sexual physiology, and similar physiological
effects can be induced by exogenous melatonin in animals including
mynah birds and hamsters. Melatonin can suppress libido by inhibiting secretion of luteinizing hormone and follicle-stimulating hormone from the anterior pituitary gland, especially in mammals that have a breeding season when daylight hours are long. The reproduction of long-day breeders is repressed by melatonin and the reproduction of short-day breeders is stimulated by melatonin.
During the night, melatonin regulates leptin, lowering its levels.
Cetaceans have lost all the genes for melatonin synthesis as well as those for melatonin receptors. This is thought to be related to their unihemispheric sleep pattern (one brain hemisphere at a time). Similar trends have been found in sirenians.
Plants
Until its
identification in plants in 1987, melatonin was for decades thought to
be primarily an animal neurohormone. When melatonin was identified in
coffee extracts in the 1970s, it was believed to be a byproduct of the
extraction process. Subsequently, however, melatonin has been found in
all plants that have been investigated. It is present in all the
different parts of plants, including leaves, stems, roots, fruits, and
seeds, in varying proportions.
Melatonin concentrations differ not only among plant species, but also
between varieties of the same species depending on the agronomic growing
conditions, varying from picograms to several micrograms per gram.
Notably high melatonin concentrations have been measured in popular
beverages such as coffee, tea, wine, and beer, and crops including corn,
rice, wheat, barley, and oats. In some common foods and beverages, including coffee and walnuts,
the concentration of melatonin has been estimated or measured to be
sufficiently high to raise the blood level of melatonin above daytime
baseline values.
Although a role for melatonin as a plant hormone has not been
clearly established, its involvement in processes such as growth and
photosynthesis is well established. Only limited evidence of endogenous
circadian rhythms in melatonin levels has been demonstrated in some
plant species and no membrane-bound receptors analogous to those known
in animals have been described. Rather, melatonin performs important
roles in plants as a growth regulator, as well as environmental stress
protector. It is synthesized in plants when they are exposed to both
biological stresses, for example, fungal infection, and nonbiological
stresses such as extremes of temperature, toxins, increased soil salinity, drought, etc.
Occurrence
Dietary supplement
Melatonin is categorized by the US Food and Drug Administration (FDA) as a dietary supplement, and is sold over-the-counter in both the US and Canada. The FDA regulations applying to medications are not applicable to melatonin.
As melatonin may cause harm in combination with certain medications or
in the case of certain disorders, a doctor or pharmacist should be
consulted before making a decision to take melatonin. In many countries, melatonin is recognized as a neurohormone and it cannot be sold over-the-counter.
Food products
Naturally-occurring melatonin has been reported in foods including tart cherries to about 0.17–13.46 ng/g, bananas and grapes, rice and cereals, herbs, plums, olive oil, wine
and beer. When birds ingest melatonin-rich plant feed, such as rice,
the melatonin binds to melatonin receptors in their brains.
When humans consume foods rich in melatonin, such as banana, pineapple,
and orange, the blood levels of melatonin increase significantly.
As reported in the New York Times in May 2011,
beverages and snacks containing melatonin were being sold in grocery
stores, convenience stores, and clubs. The FDA considered whether these
food products could continue to be sold with the label "dietary
supplements". On 13 January 2010, it issued a Warning Letter to
Innovative Beverage, creators of several beverages marketed as drinks,
stating that melatonin, while legal as a dietary supplement, was not
approved as a food additive. A different company selling a melatonin-containing beverage received a warning letter in 2015.
Commercial availability
Immediate-release
melatonin is not tightly regulated in countries where it is available
as an over-the-counter medication. It is available in doses from less
than half a milligram to 5 mg or more. Immediate-release formulations
cause blood levels of melatonin to reach their peak in about an hour.
The hormone may be administered orally, as capsules, gummies, tablets,
or liquids. It is also available for use sublingually, or as
transdermal patches.
Formerly, melatonin was derived from animal pineal tissue, such
as bovine. It is now synthetic and does not carry a risk of
contamination or the means of transmitting infectious material.
Melatonin is the most popular over-the-counter sleep remedy in
the US, resulting in sales in excess of US $400 million during 2017.
Research
A bottle of melatonin tablets. Melatonin is available in timed-release and in liquid forms.
Various uses and effects of melatonin have been studied. A 2015 review of studies of melatonin in tinnitus found the quality of evidence low, but not entirely without promise.
Headaches
Tentative evidence shows melatonin may help reduce some types of headaches including cluster and hypnic headaches.
Cancer
A 2013 review by the National Cancer Institutes found evidence for use to be inconclusive. A 2005 review of unblinded clinical trials found a reduced rate of death, but that blinded and independently conducted randomized controlled trials are needed.
Protection from radiation
Both animal and human
studies have shown melatonin to protect against radiation-induced
cellular damage. Melatonin and its metabolites protect organisms from oxidative stress by scavenging reactive oxygen species which are generated during exposure.
Nearly 70% of biological damage caused by ionizing radiation is
estimated to be attributable to the creation of free radicals,
especially the hydroxyl radical
that attacks DNA, proteins, and cellular membranes. Melatonin has been
described as a broadly protective, readily available, and orally
self-administered antioxidant that is without major known side effects.
Epilepsy
A
2016 review found no beneficial role of melatonin in reducing seizure
frequency or improving quality of life in people with epilepsy.
Secondary dysmenorrhoea
A 2016 review suggested no strong evidence of melatonin compared to placebo for dysmenorrhoea secondary to endometriosis.
Delirium
A 2016 review suggested no clear evidence of melatonin to reduce the incidence of delirium.
Gastroesophageal reflux disease
A 2011 review said melatonin is effective in relieving epigastric pain and heartburn.
Psychiatry
Melatonin might improve sleep in people with autism. Children with autism have abnormal melatonin pathways and below-average physiological levels of melatonin. Melatonin supplementation has been shown to improve sleep duration, sleep onset latency, and night-time awakenings. However, many studies on melatonin and autism rely on self-reported levels of improvement and more rigorous research is needed.
While the packaging of melatonin often warns against use in
people under 18 years of age, available studies suggest that melatonin
is an efficacious and safe treatment for insomnia in people with ADHD. However, larger and longer studies are needed to establish long-term safety and optimal dosing.
Melatonin in comparison to placebo is effective for reducing
preoperative anxiety in adults when given as premedication. It may be
just as effective as standard treatment with midazolam in reducing
preoperative anxiety. Melatonin may also reduce postoperative anxiety
(measured 6 hours after surgery) when compared to placebo.
Some supplemental melatonin users report an increase in vivid dreaming. Extremely high doses of melatonin increased REM sleep time and dream activity in people both with and without narcolepsy. Some evidence supports an antidepressant effect.