| Multi-drug-resistance tuberculosis | |
|---|---|
 | |
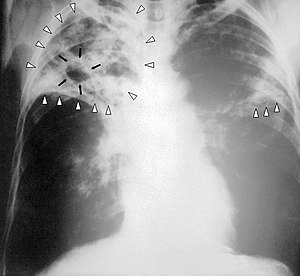
| Mycobacterium tuberculosis bacteria seen by microscope | |
| Specialty | Infectious disease |
Multi-drug-resistant tuberculosis (MDR-TB) is a form of tuberculosis (TB) infection caused by bacteria that are resistant to treatment with at least two of the most powerful first-line anti-TB medications (drugs), isoniazid and rifampin. Some forms of TB are also resistant to second-line medications, and are called extensively drug-resistant TB (XDR-TB).
Tuberculosis is caused by infection with the bacteria Mycobacterium tuberculosis. Almost one in four people in the world are infected with TB bacteria. Only when the bacteria become active do people become ill with TB. Bacteria become active as a result of anything that can reduce the person's immunity, such as HIV, advancing age, diabetes or other immunocompromising illnesses. TB can usually be treated with a course of four standard, or first-line, anti-TB drugs (i.e., isoniazid, rifampin and any fluoroquinolone).
However, beginning with the first antibiotic treatment for TB in 1943, some strains of the TB bacteria developed resistance to the standard drugs through genetic changes (see mechanisms.) Currently the majority of multidrug-resistant cases of TB are due to one strain of TB bacteria called the Beijing lineage. This process accelerates if incorrect or inadequate treatments are used, leading to the development and spread of multidrug-resistant TB (MDR-TB). Incorrect or inadequate treatment may be due to use of the wrong medications, use of only one medication (standard treatment is at least two drugs), not taking medication consistently or for the full treatment period (treatment is required for several months). Treatment of MDR-TB requires second-line drugs (i.e., fluoroquinolones, aminoglycosides, and others), which in general are less effective, more toxic and much more expensive than first-line drugs. Treatment schedules for MDR-TB involving fluoroquinolones and aminoglycosides can run for 2 years, compared to the 6 months of first-line drug treatment, and cost over US$100,000. If these second-line drugs are prescribed or taken incorrectly, further resistance can develop leading to XDR-TB.
Resistant strains of TB are already present in the population, so MDR-TB can be directly transmitted from an infected person to an uninfected person. In this case a previously untreated person develops a new case of MDR-TB. This is known as primary MDR-TB, and is responsible for up to 75% of cases. Acquired MDR-TB develops when a person with a non-resistant strain of TB is treated inadequately, resulting in the development of antibiotic resistance in the TB bacteria infecting them. These people can in turn infect other people with MDR-TB.
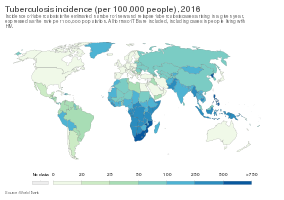
MDR-TB caused an estimated 600,000 new TB cases and 240,000 deaths in 2016 and MDR-TB accounts for 4.1% of all new TB cases and 19% of previously treated cases worldwide.[12] Globally, most MDR-TB cases occur in South America, Southern Africa, India, China, and the former Soviet Union.
Treatment of MDR-TB requires treatment with second-line drugs, usually four or more anti-TB drugs for a minimum of 6 months, and possibly extending for 18–24 months if rifampin resistance has been identified in the specific strain of TB with which the patient has been infected. Under ideal program conditions, MDR-TB cure rates can approach 70%.
Mechanism of drug resistance
The
TB bacteria has natural defenses against some drugs, and can acquire
drug resistance through genetic mutations. The bacteria does not have
the ability to transfer genes for resistance between organisms through plasmids (see horizontal transfer). Some mechanisms of drug resistance include:
- Cell wall: The cell wall of M. tuberculosis (TB) contains complex lipid molecules which act as a barrier to stop drugs from entering the cell.
- Drug modifying & inactivating enzymes: The TB genome codes for enzymes (proteins) that inactivate drug molecules. These enzymes are usually phosphorylate, acetylate, or adenylate drug compounds.
- Drug efflux systems: The TB cell contains molecular systems that actively pump drug molecules out of the cell.
- Mutations: Spontaneous mutations in the TB genome can alter proteins which are the target of drugs, making the bacteria drug resistant.
One example is a mutation in the rpoB gene, which encodes the
beta subunit of the bacteria's RNA polymerase. In non-resistant TB,
rifampin binds the beta subunit of RNA polymerase and disrupt
transcription elongation. Mutation in the rpoB gene changes the
sequence of amino acids and eventual conformation of the beta subunit.
In this case rifampin can no longer bind or prevent transcription, and
the bacteria is resistant.
Other mutations make the bacterium resistant to other drugs. For
example, there are many mutations that confer resistance to isoniazid
(INH), including in the genes katG, inhA, ahpC and
others. Amino acid replacements in the NADH binding site of InhA
apparently result in INH resistance by preventing the inhibition of
mycolic acid biosynthesis, which the bacterium uses in its cell wall.
Mutations in the katG gene make the enzyme catalase peroxidase
unable to convert INH to its biologically active form. Hence, INH is
ineffective and the bacteria is resistant. The discovery of new molecular targets is essential to overcome drug resistant problems.
In some TB bacteria, the acquisition of these mutations can be
explained other mutations in the DNA recombination, recognition and
repair machinery.
Mutations in these genes allow the bacteria to have a higher overall
mutation rate and to accumulate mutations that cause drug resistance
more quickly.
Extensively drug-resistant TB
MDR-TB can become resistant to the major second-line TB drug groups: fluoroquinolones (moxifloxacin, ofloxacin) and injectable aminoglycoside or polypeptide drugs (amikacin, capreomycin, kanamycin). When MDR-TB is resistant to at least one drug from each group, it is classified as extensively drug-resistant tuberculosis (XDR-TB).
In a study of MDR-TB patients from 2005 to 2008 in various countries, 43.7% had resistance to at least one second-line drug. About 9% of MDR-TB cases are resistant to a drug from both classes and classified as XDR-TB.
In the past 10 years TB strains have emerged in Italy, Iran,
India, and South Africa which are resistant to all available first and
second line TB drugs, classified as totally drug-resistant tuberculosis,
though there is some controversy over this term.
Increasing levels of resistance in TB strains threaten to complicate
the current global public health approaches to TB control. New drugs are
being developed to treat extensively resistant forms but major
improvements in detection, diagnosis, and treatment will be needed.
Prevention
There are several ways that drug resistance to TB, and drug resistance in general, can be prevented:
- Rapid diagnosis & treatment of TB: One of the greatest risk factors for drug resistant TB is problems in treatment and diagnosis, especially in developing countries. If TB is identified and treated soon, drug resistance can be avoided.
- Completion of treatment: Previous treatment of TB is an indicator of MDR TB. If the patient does not complete his/her antibiotic treatment, or if the physician does not prescribe the proper antibiotic regimen, resistance can develop. Also, drugs that are of poor quality or less in quantity, especially in developing countries, contribute to MDR TB.
- Patients with HIV/AIDS should be identified and diagnosed as soon as possible. They lack the immunity to fight the TB infection and are at great risk of developing drug resistance.
- Identify contacts who could have contracted TB: i.e. family members, people in close contact, etc.
- Research: Much research and funding is needed in the diagnosis, prevention and treatment of TB and MDR TB.
"Opponents of a universal tuberculosis treatment, reasoning from
misguided notions of cost-effectiveness, fail to acknowledge that MDRTB
is not a disease of poor people in distant places. The disease is
infectious and airborne. Treating only one group of patients looks
inexpensive in the short run, but will prove disastrous for all in the
long run."— Paul Farmer
DOTS-Plus
Community-based treatment programs such as DOTS-Plus, a MDR-TB-specialized treatment using the popular Directly Observed Therapy – Short Course
(DOTS) initiative, have shown considerable success in the world. In
these locales, these programs have proven to be a good option for proper
treatment of MDR-TB in poor, rural areas. A successful example has been
in Lima, Peru, where the program has seen cure rates of over 80%.
However, TB clinicians have expressed concern in the DOTS program administered in the Republic of Georgia
because it is anchored in a passive case finding. This means that the
system depends on patients coming to health care providers, without
conducting compulsory screenings. As medical anthropologists like Erin
Koch have shown, this form of implementation does not suit all cultural
structures. They urge that the DOTS protocol be constantly reformed in
the context of local practices, forms of knowledge and everyday life.
Erin Koch has used Paul Farmer's concept of "structural" violence
as a perspective for understanding how "institutions, environment,
poverty, and power reproduce, solidify, and naturalize the uneven
distribution of disease and access to resources". She has also studied
the effectiveness of the DOTS protocol in the widespread disease of
tuberculosis in the Georgian prison system.
Unlike the DOTS passive case finding used for the general Georgian
public, the multiple-level surveillance in the prison system has proven
more successful in reducing the spread of tuberculosis while increasing
rates of cure.
Koch critically notes that because the DOTS protocol aims to
change the individual's behavior without addressing the need to change
the institutional, political, and economic contexts, certain limitations
arise, such as MDR tuberculosis.
Treatment
Usually, multidrug-resistant tuberculosis can be cured with long
treatments of second-line drugs, but these are more expensive than first-line drugs and have more adverse effects.
The treatment and prognosis of MDR-TB are much more akin to those for
cancer than to those for infection. MDR-TB has a mortality rate of up to
80%, which depends on a number of factors, including:
- How many drugs the organism is resistant to (the fewer the better)
- How many drugs the patient is given (patients treated with five or more drugs do better)
- The expertise and experience of the physician responsible
- How co-operative the patient is with treatment (treatment is arduous and long, and requires persistence and determination on the part of the patient)
- Whether the patient is HIV-positive or not (HIV co-infection is associated with an increased mortality).
The majority of patients suffering from multi-drug-resistant
tuberculosis do not receive treatment, as they are found in
underdeveloped countries or in poverty. Denial of treatment remains a
difficult human rights issue, as the high cost of second-line medications often precludes those who cannot afford therapy.
A study of cost-effective strategies for tuberculosis control
supported three major policies. First, the treatment of smear-positive
cases in DOTS programs must be the foundation of any tuberculosis
control approach, and should be a basic practice for all control
programs. Second, there is a powerful economic case for treating
smear-negative and extra-pulmonary cases in DOTS programs along with
treating smear-negative and extra-pulmonary cases in DOTS programs as a
new WHO "STOP TB" approach and the second global plan for tuberculosis
control. Last, but not least, the study shows that significant scaling
up of all interventions is needed in the next 10 years if the millennium
development goal and related goals for tuberculosis control are to be
achieved. If the case detection rate can be improved, this will
guarantee that people who gain access to treatment facilities are
covered and that coverage is widely distributed to people who do not now
have access.
In general, treatment courses are measured in months to years;
MDR-TB may require surgery, and death rates remain high despite optimal
treatment. However, good outcomes for patients are still possible.
The treatment of MDR-TB must be undertaken by physicians
experienced in the treatment of MDR-TB. Mortality and morbidity in
patients treated in non-specialist centers are significantly higher to
those of patients treated in specialist centers. Treatment of MDR-TB
must be done on the basis of sensitivity testing: it is impossible to
treat such patients without this information. When treating a patient
with suspected MDR-TB, pending the result of laboratory sensitivity
testing, the patient could be started on SHREZ (Streptomycin+ isonicotinyl Hydrazine+ Rifampicin+Ethambutol+ pyraZinamide) and moxifloxacin with cycloserine.
There is evidence that previous therapy with a drug for more than a
month is associated with diminished efficacy of that drug regardless of in vitro tests indicating susceptibility.
Hence, a detailed knowledge of the treatment history of each patient is
essential. In addition to the obvious risks (i.e., known exposure to a
patient with MDR-TB), risk factors for MDR-TB include HIV infection,
previous incarceration, failed TB treatment, failure to respond to
standard TB treatment, and relapse following standard TB treatment.
A gene probe for rpoB
is available in some countries. This serves as a useful marker for
MDR-TB, because isolated RMP resistance is rare (except when patients
have a history of being treated with rifampicin alone). If the results
of a gene probe (rpoB) are known to be positive, then it is reasonable to omit RMP and to use SHEZ+MXF+cycloserine.
The reason for maintaining the patient on INH is that INH is so potent
in treating TB that it is foolish to omit it until there is
microbiological proof that it is ineffective (even though isoniazid
resistance so commonly occurs with rifampicin resistance).
For treatment of RR- and MDT-TB, WHO treatment guidelines are as
follows: "a regimen with at least five effective TB medicines during the
intensive phase is recommended, including pyrazinamide and four core
second-line TB medicines – one chosen from Group A, one from Group B,
and at least two from Group C3 (conditional recommendation, very low
certainty in the evidence). If the minimum number of effective TB
medicines cannot be composed as given above, an agent from Group D2 and
other agents from Group D3 may be added to bring the total to five. It
is recommended that the regimen be further strengthened with high-dose
isoniazid and/or ethambutol (conditional recommendation, very low
certainty in the evidence)." Medicines recommended are the following:
- Group A: Fluoroquinolones (levofloxacinm moxifloxicin, gatifloxacin), linezolid, bedaquiline
- Group B: clofazimine, cycloserine/terizidone
- Group C: Other core second-line agents (ethambutol, delamanid, pyrazinamide, imipenem-cilastatin/meropenem, amikacin/streptomycin, ethionamide/prothionamide, p-aminosalicylic acid)
For patients with RR-TB or MDR-TB, "not previously treated with
second-line drugs and in whom resistance to fluoroquinolones and
second-line injectable agents was excluded or is considered highly
unlikely, a shorter MDR-TB regimen of 9–12 months may be used instead of
the longer regimens (conditional recommendation, very low certainty in
the evidence)."
In general, resistance to one drug within a class means
resistance to all drugs within that class, but a notable exception is
rifabutin: Rifampicin-resistance does not always mean
rifabutin-resistance, and the laboratory should be asked to test for it.
It is possible to use only one drug within each drug class. If it is
difficult finding five drugs to treat then the clinician can request
that high-level INH-resistance be looked for. If the strain has only
low-level INH-resistance (resistance at 0.2 mg/l INH, but sensitive at
1.0 mg/l INH), then high dose INH can be used as part of the regimen.
When counting drugs, PZA and interferon count as zero; that is to say,
when adding PZA to a four-drug regimen, another drug must be chosen to
make five. It is not possible to use more than one injectable (STM,
capreomycin or amikacin), because the toxic effect of these drugs is
additive: If possible, the aminoglycoside should be given daily for a
minimum of three months (and perhaps thrice weekly thereafter).
Ciprofloxacin should not be used in the treatment of tuberculosis if
other fluoroquinolones are available. As of 2008, Cochrane reports that
trials of other fluoroquinolones are ongoing.
There is no intermittent regimen validated for use in MDR-TB, but
clinical experience is that giving injectable drugs for five days a
week (because there is no-one available to give the drug at weekends)
does not seem to result in inferior results. Directly observed therapy
helps to improve outcomes in MDR-TB and should be considered an integral
part of the treatment of MDR-TB.
Response to treatment must be obtained by repeated sputum
cultures (monthly if possible). Treatment for MDR-TB must be given for a
minimum of 18 months and cannot be stopped until the patient has been
culture-negative for a minimum of nine months. It is not unusual for
patients with MDR-TB to be on treatment for two years or more.
Patients with MDR-TB should be isolated in negative-pressure
rooms, if possible. Patients with MDR-TB should not be accommodated on
the same ward as immunosuppressed patients (HIV-infected patients, or
patients on immunosuppressive drugs). Careful monitoring of compliance
with treatment is crucial to the management of MDR-TB (and some
physicians insist on hospitalisation if only for this reason). Some
physicians will insist that these patients remain isolated until their
sputum is smear-negative, or even culture-negative (which may take many
months, or even years). Keeping these patients in hospital for weeks (or
months) on end may be a practical or physical impossibility, and the
final decision depends on the clinical judgement of the physician
treating that patient. The attending physician should make full use of
therapeutic drug monitoring (in particular, of the aminoglycosides) both
to monitor compliance and to avoid toxic effects.
Some supplements may be useful as adjuncts in the treatment of
tuberculosis, but, for the purposes of counting drugs for MDR-TB, they
count as zero (if four drugs are already in the regimen, it may be
beneficial to add arginine or vitamin D or both, but another drug will
be needed to make five). Supplements are:
arginine (peanuts are a good source),
vitamin D,
Dzherelo,
V5 Immunitor.
The drugs listed below have been used in desperation, and it is
uncertain as to whether they are effective at all. They are used when it
is not possible to find five drugs from the list above.
imipenem,
co-amoxiclav clofazimine,
prochlorperazine,
metronidazole.
On 28 December 2012, the U.S. Food and Drug Administration (FDA) approved bedaquiline (marketed as Sirturo by Johnson & Johnson)
to treat multi-drug resistant tuberculosis, the first new treatment in
40 years. Sirturo is to be used in a combination therapy for patients
who have failed standard treatment and have no other options. Sirturo is
an adenosine triphosphate synthase (ATP synthase) inhibitor.
The following drugs are experimental compounds that are not
commercially available, but may be obtained from the manufacturer as
part of a clinical trial or on a compassionate basis. Their efficacy and
safety are unknown:
pretomanid (manufactured by Novartis, developed in partnership with TB Alliance), and
delamanid.
In cases of extremely resistant disease, surgery to remove
infection portions of the lung is, in general, the final option. The
center with the largest experience in this is the National Jewish Medical and Research Center
in Denver, Colorado. In 17 years of experience, they have performed 180
operations; of these, 98 were lobectomies and 82 were pneumonectomies.
There is a 3.3% operative mortality, with an additional 6.8% dying
following the operation; 12% experienced significant morbidity (in
particular, extreme breathlessness). Of 91 patients who were
culture-positive before surgery, only 4 were culture-positive after
surgery.
The resurgence of tuberculosis in the United States, the advent
of HIV-related tuberculosis, and the development of strains of TB
resistant to the first-line therapies developed in recent decades—serve
to reinforce the thesis that Mycobacterium tuberculosis, the causative
organism, makes its own preferential option for the poor. The simple truth is that almost all tuberculosis deaths result from a lack of access to existing effective therapy.
Treatment success rates remain unacceptably low globally with variation between regions. 2016 data published by the WHO
reported treatment success rates of Multi-drug resistant TB globally.
For those started on treatment for multi-drug resistant TB 56%
successfully completed treatment, either treatment course completion or
eradication of disease; 15% of those died while in treatment; 15% were
lost to follow-up; 8% had treatment failure and there was no data on the
remaining 6%. Treatment success rate was highest in the World Health
Organization Mediterranean region at 65%. Treatment success rates were
lower than 50% in the Ukraine, Mozambique, Indonesia and India. Areas
with poor TB surveillance infrastructure had higher rates of loss to
follow-up of treatment.
57 countries reported outcomes for patients started on
extreme-drug resistant Tuberculosis, this included 9258 patients. 39%
completed treatment successfully, 26% of patients died and treatment
failed for 18%. 84% of the extreme Drug resistant Cohort was made up of
only three countries; India, Russian Federation and Ukraine. Shorter
treatment regimes for MDR-TB have been found to be beneficial having
higher treatment success rates.
Epidemiology
Cases of MDR tuberculosis have been reported in every country surveyed. MDR-TB most commonly develops in the course of TB treatment,
and is most commonly due to doctors giving inappropriate treatment, or
patients missing doses or failing to complete their treatment. Because
MDR tuberculosis is an airborne pathogen, persons with active, pulmonary
tuberculosis caused by a multidrug-resistant strain can transmit the
disease if they are alive and coughing.
TB strains are often less fit and less transmissible, and outbreaks
occur more readily in people with weakened immune systems (e.g.,
patients with HIV). Outbreaks among non immunocompromised healthy people do occur, but are less common.
As of 2013, 3.7% of new tuberculosis cases have MDR-TB. Levels
are much higher in those previously treated for tuberculosis - about
20%. WHO estimates that there were about 0.5 million new MDR-TB cases in
the world in 2011. About 60% of these cases occurred in Brazil, China,
India, the Russian Federation and South Africa alone. In Moldova, the crumbling health system has led to the rise of MDR-TB. In 2013, the Mexico–United States border was noted to be "a very hot region for drug resistant TB", though the number of cases remained small.
It has been known for many years that INH-resistant TB is less
virulent in guinea pigs, and the epidemiological evidence is that MDR
strains of TB do not dominate naturally. A study in Los Angeles,
California, found that only 6% of cases of MDR-TB were clustered.
Likewise, the appearance of high rates of MDR-TB in New York City in the
early 1990s was associated with the explosion of AIDS in that area.
In New York City, a report issued by city health authorities states
that fully 80 percent of all MDR-TB cases could be traced back to
prisons and homeless shelters.
When patients have MDR-TB, they require longer periods of
treatment—about two years of multidrug regimen. Several of the less
powerful second-line drugs, which are required to treat MDR-TB, are also
more toxic, with side effects such as nausea, abdominal pain, and even
psychosis. The Partners in Health team had treated patients in Peru who
were sick with strains that were resistant to ten and even twelve drugs.
Most such patients require adjuvant surgery for any hope of a cure.
Somalia
MDR-TB
is widespread in Somalia, where 8.7% of newly discovered TB cases are
restistant to Rifampicin and Isoniazid, in patients which were treated
previously the share was 47%.
Refugees from Somalia brought an until then unknown variant of
MDR tuberculosis with them to Europe. A few number of cases in four
different countries were considered by the European Centre for Disease Prevention and Control to pose no risk to the native population.
Russian prisons
One of the so-called "hot-spots" of drug-resistant tuberculosis is within the Russian prison system.
Infectious disease researchers Nachega & Chaisson report that 10%
of the one million prisoners within the system have active TB.
One of their studies found that 75% of newly diagnosed inmates with TB
are resistant to at least one drug; 40% of new cases are multi-drug
resistant.
In 1997, TB accounted for almost half of all Russian prison deaths, and
as Bobrik et al. point out in their public health study, the 90%
reduction in TB incidence contributed to a consequential fall in the
prisoner death rate in the years following 1997.
Baussano et al. articulate that concerning statistics like these are
especially worrisome because spikes in TB incidence in prisons are
linked to corresponding outbreaks in surrounding communities.
Additionally, rising rates of incarceration, especially in Central
Asian and Eastern European countries like Russia, have been correlated
with higher TB rates in civilian populations. Even as the DOTS program
is expanded throughout Russian prisons, researchers such as Shin et al.
have noted that wide-scale interventions have not had their desired
effect, especially with regard to the spread of drug-resistant strains
of TB.
Contributing factors
There
are several elements of the Russian prison system that enable the
spread of MDR-TB and heighten its severity. Overcrowding in prisons is
especially conducive to the spread of tuberculosis; an inmate in a
prison hospital has (on average) 3 meters of personal space, and an
inmate in a correctional colony has 2 meters.
Specialized hospitals and treatment facilities within the prison
system, known as TB colonies, are intended to isolate infected prisoners
to prevent transmission; however, as Ruddy et al. demonstrate, there
are not enough of these colonies to sufficiently protect staff and other
inmates.
Additionally, many cells lack adequate ventilation, which increases
likelihood of transmission. Bobrik et al. have also noted food shortages
within prisons, which deprive inmates of the nutrition necessary for
healthy functioning.
Comorbidity of HIV
within prison populations has also been shown to worsen health
outcomes. Nachega & Chaisson articulate that while HIV-infected
prisoners are not more susceptible MDR-TB infection, they are more
likely to progress to serious clinical illness if infected. According to Stern, HIV infection is 75 times more prevalent in Russian prison populations than in the civilian population.
Therefore, prison inmates are both more likely to become infected with
MDR-TB initially and to experience severe symptoms because of previous
exposure to HIV.
Shin et al. emphasize another factor in MDR-TB prevalence in Russian prisons: alcohol and substance use. Ruddy et al. showed that risk for MDR-TB is three times higher among recreational drug users than non-users.
Shin et al.'s study demonstrated that alcohol usage was linked to
poorer outcomes in MDR-TB treatment; they also noted that a majority of
subjects within their study (many of whom regularly used alcohol) were
nevertheless cured by their aggressive treatment regimen.
Non-compliance with treatment plans is often cited as a
contributor to MDR-TB transmission and mortality. Indeed, of the 80
newly-released TB-infected inmates in Fry et al.'s study, 73.8% did not
report visiting a community dispensary for further treatment.
Ruddy et al. cite release from facilities as one of the main causes of
interruption in prisoner's TB treatment, in addition to non-compliance
within the prison and upon reintegration into civilian life.
Fry et al.'s study also listed side effects of TB treatment medications
(especially in HIV positive individuals), financial worries, housing
insecurities, family problems, and fear of arrest as factors that
prevented some prisoners from properly adhering to TB treatment.
They also note that some researchers have argued that the short-term
gains TB-positive prisoners receive, such as better food or work
exclusion, may dis-incentivize becoming cured.
In their World Health Organization article, Gelmanova et al. posit that
non-adherence to TB treatment indirectly contributes to bacterial
resistance.
Although ineffective or inconsistent treatment does not "create"
resistant strains, mutations within the high bacterial load in
non-adherent prisoners can cause resistance.
Nachega & Chaisson argue that inadequate TB control programs are the strongest driver of MDR-TB incidence. They note that prevalence of MDR-TB is 2.5 times higher in areas of poorly controlled TB.
Russian-based therapy (i.e., not DOTS) has been criticized by Kimerling
et al. as "inadequate" in properly controlling TB incidence and
transmission.
Bobrik et al. note that treatment for MDR-TB is equally inconsistent;
the second-line drugs used to treat the prisoners lack specific
treatment guidelines, infrastructure, training, or follow-up protocols
for prisoners reentering civilian life.
Policy impacts
As
Ruddy et al. note in their scholarly article, Russia's recent penal
reforms will greatly reduce the number of inmates inside prison
facilities and thus increase the number of ex-convicts integrated into
civilian populations.
Because the incidence of MDR-TB is strongly predicted by past
imprisonment, the health of Russian society will be greatly impacted by
this change.
Formerly incarcerated Russians will re-enter civilian life and remain
within that sphere; as they live as civilians, they will infect others
with the contagions they were exposed to in prison. Researcher Vivian
Stern argues that the risk of transmission from prison populations to
the general public calls for an integration of prison healthcare and
national health services to better control both TB and MDR-TB.
While second-line drugs necessary for treating MDR-TB are arguably more
expensive than a typical regimen of DOTS therapy, infectious disease
specialist Paul Farmer
posits that the outcome of leaving infected prisoners untreated could
cause a massive outbreak of MDR-TB in civilian populations, thereby
inflicting a heavy toll on society. Additionally, as MDR-TB spreads, the threat of the emergence of totally-drug-resistant TB becomes increasingly apparent.