From Wikipedia, the free encyclopedia
 |
|||
| Names | |||
|---|---|---|---|
| IUPAC name
Azane
|
|||
| Other names
Hydrogen nitride
Trihydrogen nitride Nitro-Sil |
|||
| Identifiers | |||
| 3DMet | B00004 | ||
| 3587154 | |||
| CAS number | 7664-41-7 |
||
| ChEBI | CHEBI:16134 |
||
| ChEMBL | ChEMBL1160819 |
||
| ChemSpider | 217 |
||
| EC number | 231-635-3 | ||
| 79 | |||
|
|||
| Jmol-3D images | Image | ||
| KEGG | D02916 |
||
| MeSH | Ammonia | ||
| PubChem | 222 | ||
| RTECS number | BO0875000 | ||
|
|||
| UNII | 5138Q19F1X |
||
| UN number | 1005 | ||
| Properties | |||
| NH3 | |||
| Molar mass | 17.031 g/mol | ||
| Appearance | Colourless gas | ||
| Odor | strong pungent odor | ||
| Density | 0.86 kg/m3 (1.013 bar at boiling point) 0.769 kg/m3 (STP)[1] 0.73 kg/m3 (1.013 bar at 15 °C) 681.9 kg/m3 at −33.3 °C (liquid)[2] 817 kg/m3 at −80 °C (transparent solid)[3] |
||
| Melting point | −77.73 °C (−107.91 °F; 195.42 K) | ||
| Boiling point | −33.34 °C (−28.01 °F; 239.81 K) | ||
| 47% w/w (0 °C) 31% w/w (25 °C) 18% w/w (50 °C)[4] |
|||
| Solubility | soluble in chloroform, ether, ethanol, methanol | ||
| Vapor pressure | 8573 h Pa | ||
| Acidity (pKa) | 32.5 (−33 °C),[5] 10.5 (DMSO) | ||
| Basicity (pKb) | 4.75 | ||
Refractive index (nD)
|
1.3327 | ||
| Structure | |||
| Point group | C3v | ||
| Molecular shape | Trigonal pyramid | ||
| Dipole moment | 1.42 D | ||
| Thermochemistry | |||
Std molar
entropy (S |
193 J·mol−1·K−1[6] | ||
Std enthalpy of
formation (ΔfH |
−46 kJ·mol−1[6] | ||
| Hazards | |||
| MSDS | External MSDS | ||
| GHS pictograms |     [7] [7] |
||
| H221, H280, H314, H331, H400[7] | |||
| P210, P261, P273, P280, P305+351+338, P310[7] | |||
| EU Index | 007-001-00-5 (anhydrous) 007-001-01-2 (solutions) |
||
| EU classification | |||
| R-phrases | R10, R23, R34, R50 | ||
| S-phrases | (S1/2), S9, S16, S26, S36/37/39, S45, S61 | ||
| NFPA 704 | |||
| Flash point | flammable gas | ||
| 651 °C (1,204 °F; 924 K) | |||
| Explosive limits | 15–28% | ||
LD50 (Lethal dose)
|
0.015 mL/kg (human, oral) | ||
| US health exposure limits (NIOSH):[8] | |||
PEL (Permissible)
|
50 ppm (25 ppm ACGIH- TLV; 35 ppm STEL) | ||
REL (Recommended)
|
TWA 25 ppm (18 mg/m3) ST 35 ppm (27 mg/m3) | ||
LDLH (Immediate danger)
|
300 ppm | ||
| Related compounds | |||
Other cations
|
Phosphine Arsine Stibine |
||
Related nitrogen hydrides
|
Hydrazine Hydrazoic acid |
||
Related compounds
|
Ammonium hydroxide | ||
| Supplementary data page | |||
| Refractive index (n), Dielectric constant (εr), etc. |
|||
Thermodynamic
data |
Phase behaviour solid–liquid–gas |
||
| UV, IR, NMR, MS | |||
Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa)
|
|||
NH3 boils at −33.34 °C (−28.012 °F) at a pressure of one atmosphere, so the liquid must be stored under pressure or at low temperature. Household ammonia or ammonium hydroxide is a solution of NH3 in water. The concentration of such solutions is measured in units of the Baumé scale (density), with 26 degrees baumé (about 30% (by weight) ammonia at 15.5 °C or 59.9 °F) being the typical high-concentration commercial product.[11]
Natural occurrence
Ammonia is found in trace quantities in the atmosphere, being produced from the putrefaction (decay process) of nitrogenous animal and vegetable matter. Ammonia and ammonium salts are also found in small quantities in rainwater, whereas ammonium chloride (sal-ammoniac), and ammonium sulfate are found in volcanic districts; crystals of ammonium bicarbonate have been found in Patagonian guano. The kidneys secrete ammonia to neutralize excess acid.[12] Ammonium salts are found distributed through fertile soil and in seawater. Ammonia is also found throughout the Solar System on Pluto, Mars, Jupiter, Saturn, Uranus, and Neptune. Substances containing ammonia, or those that are similar to it, are called ammoniacal.Properties
Ammonia is a colourless gas with a characteristic pungent smell. It is lighter than air, its density being 0.589 times that of air. It is easily liquefied due to the strong hydrogen bonding between molecules; the liquid boils at −33.3 °C (−27.94 °F), and freezes at −77.7 °C (−107.86 °F) to white crystals.[13]Ammonia may be conveniently deodorized by reacting it with either sodium bicarbonate or acetic acid. Both of these reactions form an odorless ammonium salt.
- Solid
- The crystal symmetry is cubic, Pearson symbol cP16, space group P213 No.198, lattice constant 0.5125 nm.[14]
- Liquid
- Liquid ammonia possesses strong ionising powers reflecting its high ε of 22. Liquid ammonia has a very high standard enthalpy change of vaporization (23.35 kJ/mol, cf. water 40.65 kJ/mol, methane 8.19 kJ/mol, phosphine 14.6 kJ/mol) and can therefore be used in laboratories in uninsulated vessels without additional refrigeration. See liquid ammonia as a solvent.
- Solvent properties
- Ammonia is miscible with water. In an aqueous solution, it can be expelled by boiling. The aqueous solution of ammonia is basic. The maximum concentration of ammonia in water (a saturated solution) has a density of 0.880 g/cm3 and is often known as '.880 ammonia'. Ammonia does not burn readily or sustain combustion, except under narrow fuel-to-air mixtures of 15–25% air.
- Combustion
- When mixed with oxygen, it burns with a pale yellowish-green flame. At high temperature and in the presence of a suitable catalyst, ammonia is decomposed into its constituent elements. Ignition occurs when chlorine is passed into ammonia, forming nitrogen and hydrogen chloride; if chlorine is present in excess, then the highly explosive nitrogen trichloride (NCl3) is also formed.
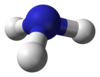
Structure
The ammonia molecule has a trigonal pyramidal shape as predicted by the valence shell electron pair repulsion theory (VSEPR theory) with an experimentally determined bond angle of 106.7°.[15] The central nitrogen atom has five outer electrons with an additional electron from each hydrogen atom. This gives a total of eight electrons, or four electron pairs that are arranged tetrahedrally. Three of these electron pairs are used as bond pairs, which leaves one lone pair of electrons. The lone pair of electrons repel more strongly than bond pairs, therefore the bond angle is not 109.5°, as expected for a regular tetrahedral arrangement, but is measured at 106.7°.[15] The nitrogen atom in the molecule has a lone electron pair, which makes ammonia a base, a proton acceptor. This shape gives the molecule a dipole moment and makes it polar. The molecule's polarity and, especially, its ability to form hydrogen bonds, makes ammonia highly miscible with water. Ammonia is moderately basic, a 1.0 M aqueous solution has a pH of 11.6 and if a strong acid is added to such a solution until the solution is neutral (pH = 7), 99.4% of the ammonia molecules are protonated. Temperature and salinity also affect the proportion of NH4+. The latter has the shape of a regular tetrahedron and is isoelectronic with methane.The ammonia molecule readily undergoes nitrogen inversion at room temperature; a useful analogy is an umbrella turning itself inside out in a strong wind. The energy barrier to this inversion is 24.7 kJ/mol, and the resonance frequency is 23.79 GHz, corresponding to microwave radiation of a wavelength of 1.260 cm. The absorption at this frequency was the first microwave spectrum to be observed.[16]
Amphotericity
One of the most characteristic properties of ammonia is its basicity. Ammonia is considered to be a weak base. It combines with acids to form salts; thus with hydrochloric acid it forms ammonium chloride (sal-ammoniac); with nitric acid, ammonium nitrate, etc. However, perfectly dry ammonia will not combine with perfectly dry hydrogen chloride; moisture is necessary to bring about the reaction.[17] As a demonstration experiment, opened bottles of concentrated ammonia and hydrochloric acid produce clouds of ammonium chloride, which seem to appear "out of nothing" as the salt forms where the two diffusing clouds of molecules meet, somewhere between the two bottles.- NH3 + HCl → NH4Cl
Although ammonia is well known as a weak base, it can also act as an extremely weak acid. It is a protic substance and is capable of formation of amides (which contain the NH2− ion). For example, lithium dissolves in liquid ammonia to give a solution of lithium amide:
- Li + NH3 → LiNH2 + ½ H2
Self-dissociation
Like water, ammonia undergoes molecular autoionisation to form its acid and base conjugates:- 2 NH
3 (aq) NH+
NH+
4 (aq) + NH−
2 (aq)
4][NH−
2] = 10−30
Combustion
The combustion of ammonia to nitrogen and water is exothermic:- 4 NH3 + 3 O2 → 2 N2 + 6 H2O (g) (ΔH°r = −1267.20 kJ/mol)
- 4 NH3 + 5 O2 → 4 NO + 6 H2O
- 2 NO + O2 → 2 NO2
Formation of other compounds
In organic chemistry, ammonia can act as a nucleophile in substitution reactions. Amines can be formed by the reaction of ammonia with alkyl halides, although the resulting -NH2 group is also nucleophilic and secondary and tertiary amines are often formed as byproducts. An excess of ammonia helps minimise multiple substitution, and neutralises the hydrogen halide formed.Methylamine is prepared commercially by the reaction of ammonia with chloromethane, and the reaction of ammonia with 2-bromopropanoic acid has been used to prepare racemic alanine in 70% yield. Ethanolamine is prepared by a ring-opening reaction with ethylene oxide: the reaction is sometimes allowed to go further to produce diethanolamine and triethanolamine.
Amides can be prepared by the reaction of ammonia with a number of carboxylic acid derivatives. Acyl chlorides are the most reactive, but the ammonia must be present in at least a twofold excess to neutralise the hydrogen chloride formed. Esters and anhydrides also react with ammonia to form amides. Ammonium salts of carboxylic acids can be dehydrated to amides so long as there are no thermally sensitive groups present: temperatures of 150–200 °C are required.
The hydrogen in ammonia is capable of replacement by metals, thus magnesium burns in the gas with the formation of magnesium nitride Mg3N2, and when the gas is passed over heated sodium or potassium, sodamide, NaNH2, and potassamide, KNH2, are formed. Where necessary in substitutive nomenclature, IUPAC recommendations prefer the name "azane" to ammonia: hence chloramine would be named "chloroazane" in substitutive nomenclature, not "chloroammonia".
Pentavalent ammonia is known as λ5-amine, or more commonly, ammonium hydride. This crystalline solid is only stable under high pressure, and decomposes back into trivalent ammonia and hydrogen gas at normal conditions. This substance was once investigated as a possible solid rocket fuel in 1966.[19]
Ammonia as a ligand

Ball-and-stick model of the tetraamminediaquacopper(II) cation, [Cu(NH3)4(H2O)2]2+
Ammonia can act as a ligand in transition metal complexes. It is a pure σ-donor, in the middle of the spectrochemical series, and shows intermediate hard-soft behaviour. For historical reasons, ammonia is named ammine in the nomenclature of coordination compounds. Some notable ammine complexes include tetraamminediaquacopper(II) ([Cu(NH3)4(H2O)2]2+), a dark blue complex formed by adding ammonia to solution of copper(II) salts. It is known as Schweizer's reagent. Diamminesilver(I) ([Ag(NH3)2]+) is the active species in Tollens' reagent. Formation of this complex can also help to distinguish between precipitates of the different silver halides: silver chloride (AgCl) is soluble in dilute (2M) ammonia solution, silver bromide (AgBr) is only soluble in concentrated ammonia solution, whereas silver iodide (AgI) is insoluble in aqueous ammonia.
Ammine complexes of chromium(III) were known in the late 19th century, and formed the basis of Alfred Werner's revolutionary theory on the structure of coordination compounds. Werner noted only two isomers (fac- and mer-) of the complex [CrCl3(NH3)3] could be formed, and concluded the ligands must be arranged around the metal ion at the vertices of an octahedron. This proposal has since been confirmed by X-ray crystallography.
An ammine ligand bound to a metal ion is markedly more acidic than a free ammonia molecule, although deprotonation in aqueous solution is still rare. One example is the Calomel reaction, where the resulting amidomercury(II) compound is highly insoluble.
- Hg2Cl2 + 2 NH3 → Hg + HgCl(NH2) + NH4+ + Cl−
Ammonia in Group Theory
The point group for ammonia is C3v when the major axis is through the nitrogen vertically. When the major axis is spun either clockwise or counterclockwise by 120°, each hydrogen is moved into the previous location of another hydrogen. The other aspect of the C3v point group included 3 vertical planes of symmetry that transects the nitrogen and one of the hydrogens allowing the other two hydrogens to be reflected into one another.The matrix math for this particular subgroup is complicated since the matrices produced from performing the rotations or the reflections are reducible compared to other matrices that are irreducible. Because of this, a similarity transformation must be performed on each portion of the matrix that is reducible. The similarity transformation for ammonia comes from the molecular orbital diagram's symmetry adapted linear combination (SALC) calculations for the contribution of bonding from each of the hydrogens.
Detection and determination
Ammonia in solution
Ammonia and ammonium salts can be readily detected, in very minute traces, by the addition of Nessler's solution, which gives a distinct yellow colouration in the presence of the least trace of ammonia or ammonium salts. The amount of ammonia in ammonium salts can be estimated quantitatively by distillation of the salts with sodium or potassium hydroxide, the ammonia evolved being absorbed in a known volume of standard sulfuric acid and the excess of acid then determined volumetrically; or the ammonia may be absorbed in hydrochloric acid and the ammonium chloride so formed precipitated as ammonium hexachloroplatinate, (NH4)2PtCl6.Gaseous ammonia
Sulfur sticks are burnt to detect small leaks in industrial ammonia refrigeration systems. Larger quantities can be detected by warming the salts with a caustic alkali or with quicklime, when the characteristic smell of ammonia will be at once apparent. Ammonia is an irritant and irritation increases with concentration; the Permissible Exposure Limit is 25 ppm, and lethal above 500 ppm.[20] Higher concentrations are hardly detected by conventional detectors, the type of detector is chosen according to the sensitivity required (e.g. semiconductor, catalytic, electrochemical). Holographic sensors have been proposed for detecting concentrations up to 12.5% in volume.[21]Ammoniacal nitrogen (NH3-N)
Ammoniacal nitrogen (NH3-N) is a measure commonly used for testing the quantity of ammonium ions, derived naturally from ammonia, and returned to ammonia via organic processes, in water or waste liquids. It is a measure used mainly for quantifying values in waste treatment and water purification systems, as well as a measure of the health of natural and man made water reserves. It is measured in units of mg/L (milligram per liter).History

This high-pressure reactor was built in 1921 by BASF in Ludwigshafen and was re-erected on the premises of the University of Karlsruhe in Germany.
The Romans gave the name sal ammoniacus (salt of Amun) to the ammonium chloride deposits they collected from near the Temple of Amun (Greek Ἄμμων Ammon) in ancient Libya because of proximity to the nearby temple.[22] Salts of ammonia have been known from very early times; thus the term Hammoniacus sal appears in the writings of Pliny,[23] although it is not known whether the term is identical with the more modern sal-ammoniac (ammonium chloride).[24]
In the form of sal-ammoniac (نشادر, nushadir) ammonia was important to the Muslim alchemists as early as the 8th century, first mentioned by the Persian chemist Jābir ibn Hayyān,[25] and to the European alchemists since the 13th century, being mentioned by Albertus Magnus.[13] It was also used by dyers in the Middle Ages in the form of fermented urine to alter the colour of vegetable dyes. In the 15th century, Basilius Valentinus showed that ammonia could be obtained by the action of alkalis on sal-ammoniac. At a later period, when sal-ammoniac was obtained by distilling the hooves and horns of oxen and neutralizing the resulting carbonate with hydrochloric acid, the name "spirit of hartshorn" was applied to ammonia.[13][26]
Gaseous ammonia was first isolated by Joseph Priestley in 1774 and was termed by him "alkaline air".[27] Eleven years later in 1785, Claude Louis Berthollet ascertained its composition.[13]
The Haber–Bosch process to produce ammonia from the nitrogen in the air was developed by Fritz Haber and Carl Bosch in 1909 and patented in 1910. It was first used on an industrial scale in Germany during World War I,[10] following the allied blockade that cut off the supply of nitrates from Chile. The ammonia was used to produce explosives to sustain war efforts.[28]
Prior to the availability of cheap natural gas, hydrogen as a precursor to ammonia production was produced via the electrolysis of water or using the chloralkali process.
Uses
Fertilizer
Approximately 83% (as of 2004) of ammonia is used as fertilizers either as its salts, solutions or anhydrously. When applied to soil, it helps provide increased yields of crops such as maize and wheat.[citation needed] 30% of agricultural nitrogen applied in the USA is in the form of anhydrous ammonia and worldwide 110 million tonnes are applied each year.[29]Precursor to nitrogenous compounds
Ammonia is directly or indirectly the precursor to most nitrogen-containing compounds. Virtually all synthetic nitrogen compounds are derived from ammonia. An important derivative is nitric acid. This key material is generated via the Ostwald process by oxidation of ammonia with air over a platinum catalyst at 700–850 °C (1,292–1,562 °F), ~9 atm. Nitric oxide is an intermediate in this conversion:[30]- NH3 + 2 O2 → HNO3 + H2O
Ammonia is also used to make the following compounds:
- Hydrazine, in the Olin Raschig process and the peroxide process
- Hydrogen cyanide, in the BMA process and the Andrussow process
- Hydroxylamine and ammonium carbonate, in the Raschig process
- Phenol, in the Raschig–Hooker process
- Urea, in the Bosch–Meiser urea process and in Wöhler synthesis
- Amino acids, using Strecker amino-acid synthesis
- Acrylonitrile, in the Sohio process
Examples of such compounds include: ammonium perchlorate, ammonium nitrate, formamide, dinitrogen tetroxide, alprazolam, ethanolamine, ethyl carbamate, hexamethylenetetramine, and ammonium bicarbonate.
Cleaner
Household ammonia is a solution of NH3 in water (i.e., ammonium hydroxide) used as a general purpose cleaner for many surfaces. Because ammonia results in a relatively streak-free shine, one of its most common uses is to clean glass, porcelain and stainless steel. It is also frequently used for cleaning ovens and soaking items to loosen baked-on grime. Household ammonia ranges in concentration by weight from 5 to 10% ammonia.Fermentation
Solutions of ammonia ranging from 16% to 25% are used in the fermentation industry as a source of nitrogen for microorganisms and to adjust pH during fermentation.Antimicrobial agent for food products
As early as in 1895, it was known that ammonia was "strongly antiseptic ... it requires 1.4 grams per litre to preserve beef tea."[31] In one study, anhydrous ammonia destroyed 99.999% of zoonotic bacteria in 3 types of animal feed, but not silage.[32][non-primary source needed] Anhydrous ammonia is currently used commercially to reduce or eliminate microbial contamination of beef.[33][34] Pink slime (or lean finely textured beef in the beef industry) is made from fatty beef trimmings (about 50–70% fat) by removing the fat using heat and centrifugation, then treating it with ammonia to kill E. coli. The process was deemed effective and safe by the US Department of Agriculture based on a study (funded by a producer of pink slime) that found that the treatment reduces E. coli to undetectable levels.[35] There have been safety concerns about the process as well as consumer complaints about the taste and smell of beef treated at optimal levels of ammonia.[36] However, the level of ammonia in any final product has not come close to toxic levels to humans.Minor and emerging uses
Refrigeration – R717
Because of ammonia's vaporization properties, it is a useful refrigerant.[10] It was commonly used prior to the popularisation of chlorofluorocarbons (Freons). Anhydrous ammonia is widely used in industrial refrigeration applications and hockey rinks because of its high energy efficiency and low cost. However, it suffers from the disadvantage of toxicity, which restricts its domestic and small-scale use. Along with its use in modern vapor-compression refrigeration it was used in a mixture along with hydrogen and water in absorption refrigerators. The Kalina cycle, which is of growing importance to geothermal power plants, depends on the wide boiling range of the ammonia-water mixture.For remediation of gaseous emissions
Ammonia is used to scrub SO2 from the burning of fossil fuels, and the resulting product is converted to ammonium sulfate for use as fertilizer. Ammonia neutralizes the nitrogen oxides (NOx) pollutants emitted by diesel engines. This technology, called SCR (selective catalytic reduction), relies on a vanadia-based catalyst.[37]Ammonia may be used to mitigate gaseous spills of phosgene.[38]
As a fuel
Ammonia was used during World War II to power buses in Belgium, and in engine and solar energy applications prior to 1900. Liquid ammonia also fuelled the Reaction Motors XLR99 rocket engine that powered the X-15 hypersonic research aircraft. Although not as powerful as other fuels, it left no soot in the reusable rocket engine and its density approximately matches the density of the oxidizer, liquid oxygen, which simplified the aircraft's design.
Ammonia has been proposed as a practical alternative to fossil fuel for internal combustion engines.[39] The calorific value of ammonia is 22.5 MJ/kg (9690 BTU/lb), which is about half that of diesel. In a normal engine, in which the water vapour is not condensed, the calorific value of ammonia will be about 21% less than this figure.
Ammonia cannot be easily or efficiently used in existing Otto cycle engines because of its very low octane rating, although with only minor modifications to carburettors/injectors and a drastic reduction in compression ratio, which would require new pistons, a gasoline engine could be made to work exclusively with ammonia, at a low fraction of its power output before conversion and much higher fuel consumption.[citation needed]
An automobile fuel tank could store ammonia as a liquid as long as the tank was pressurized appropriately, depending on the temperature. Ammonia's thermodynamic properties are such that at -30 C, the tank pressure would only have to be 27.5 psi, about the same as a car tire. At 30 °C (86 °F) the pressure in the tank would need to be 170 psi to keep the ammonia liquid. If tank pressure was released, the liquid ammonia would turn gaseous and raise the pressure again to that level. Common pneumatic tool air compressors operate at this pressure, so fuel tank pressure is not a barrier to adoption of automobile fuel usage.
However, there are other barriers to widespread automobile usage. In terms of raw ammonia supplies, plants would have to be built to increase production levels, requiring significant capital and energy sources. Although it is the second most produced chemical, the scale of ammonia production is a small fraction of world petroleum usage. It could be manufactured from renewable energy sources, as well as coal or nuclear power. The 60 MW Rjukan dam in Telemark, Norway produced ammonia via electrolysis of water for many years from 1913 producing fertilizer for much of Europe. If produced from coal, the CO2 could be sequestered, but carbon capture and storage from coal power plants is not yet beyond prototype stages.
In 1981, a Canadian company converted a 1981 Chevrolet Impala to operate using ammonia as fuel.[40][41]
In 2007, a University of Michigan pickup powered by ammonia drove from Detroit to San Francisco as part of a demonstration, requiring only one fill-up in Wyoming.[42]
Ammonia engines or ammonia motors, using ammonia as a working fluid, have been proposed and occasionally used.[43] The principle is similar to that used in a fireless locomotive, but with ammonia as the working fluid, instead of steam or compressed air. Ammonia engines were used experimentally in the 19th century by Goldsworthy Gurney in the UK and in streetcars in New Orleans.
As a stimulant

Anti-meth sign on tank of anhydrous ammonia, Otley, Iowa. Anhydrous ammonia is a common farm fertilizer that is also a critical ingredient in making methamphetamine. In 2005, Iowa state used grant money to give out thousands of locks to prevent criminals from getting into the tanks.[44]
Ammonia, as the vapor released by smelling salts, has found significant use as a respiratory stimulant. Ammonia is commonly used in the illegal manufacture of methamphetamine through a Birch reduction.[45] The Birch method of making methamphetamine is dangerous because the alkali metal and liquid ammonia are both extremely reactive, and the temperature of liquid ammonia makes it susceptible to explosive boiling when reactants are added.[citation needed]
Textile
Liquid ammonia is used for treatment of cotton materials, giving properties like mercerisation, using alkalis. In particular, it is used for prewashing of wool.[46]Lifting gas
At standard temperature and pressure, ammonia is less dense than atmosphere, and has approximately 60% of the lifting power of hydrogen or helium. Ammonia has sometimes been used to fill weather balloons as a lifting gas. Because of its relatively high boiling point (compared to helium and hydrogen), ammonia could potentially be refrigerated and liquefied aboard an airship to reduce lift and add ballast (and returned to a gas to add lift and reduce ballast).Woodworking
Ammonia has been used to darken quartersawn white oak in Arts & Crafts and Mission-style furniture. Ammonia fumes react with the natural tannins in the wood and cause it to change colours.[47]Safety precautions

The world's longest ammonia pipeline, running from the TogliattiAzot plant in Russia to Odessa in Ukraine.
The U. S. Occupational Safety and Health Administration (OSHA) has set a 15-minute exposure limit for gaseous ammonia of 35 ppm by volume in the environmental air and an 8-hour exposure limit of 25 ppm by volume.[48] NIOSH recently reduced the IDLH from 500 to 300 based on recent more conservative interpretations of original research in 1943. IDLH (Immediately Dangerous to Life and Health) is the level to which a healthy worker can be exposed for 30 minutes without suffering irreversible health effects. Other organizations have varying exposure levels. U.S. Navy Standards [U.S. Bureau of Ships 1962] maximum allowable concentrations (MACs):continuous exposure (60 days): 25 ppm / 1 hour: 400 ppm[49] Ammonia vapour has a sharp, irritating, pungent odour that acts as a warning of potentially dangerous exposure. The average odour threshold is 5 ppm, well below any danger or damage. Exposure to very high concentrations of gaseous ammonia can result in lung damage and death.[48] Although ammonia is regulated in the United States as a non-flammable gas, it still meets the definition of a material that is toxic by inhalation and requires a hazardous safety permit when transported in quantities greater than 13,248 L (3,500 gallons).[50]
Toxicity
The toxicity of ammonia solutions does not usually cause problems for humans and other mammals, as a specific mechanism exists to prevent its build-up in the bloodstream. Ammonia is converted to carbamoyl phosphate by the enzyme carbamoyl phosphate synthetase, and then enters the urea cycle to be either incorporated into amino acids or excreted in the urine[citation needed]. However, fish and amphibians lack this mechanism, as they can usually eliminate ammonia from their bodies by direct excretion. Ammonia even at dilute concentrations is highly toxic to aquatic animals, and for this reason it is classified as dangerous for the environment.Aquaculture
Ammonia toxicity is believed to be a cause of otherwise unexplained losses in fish hatcheries. Excess ammonia may accumulate and cause alteration of metabolism or increases in the body pH of the exposed organism. Tolerance varies among fish species.[51] At lower concentrations, around 0.05 mg/L, un-ionised ammonia is harmful to fish species and can result in poor growth and feed conversion rates, reduced fecundity and fertility and increase stress and susceptibility to bacterial infections and diseases.[52] Exposed to excess ammonia, fish may suffer loss of equilibrium, hyper-excitability, increased respiratory activity and oxygen uptake and increased heart rate.[51] At concentrations exceeding 2.0 mg/L, ammonia causes gill and tissue damage, extreme lethargy, convulsions, coma, and death.[51][53] Experiments have shown that the lethal concentration for a variety of fish species ranges from 0.2 to 2.0 mg/l.[53]During winter, when reduced feeds are administered to aquaculture stock, ammonia levels can be higher. Lower ambient temperatures reduce the rate of algal photosynthesis so less ammonia is removed by any algae present. Within an aquaculture environment, especially at large scale, there is no fast acting remedy to elevated ammonia levels. Prevention rather than correction is recommended to reduce harm to farmed fish[53] and in open water systems, the surrounding environment.
Storage information
Similar to propane, anhydrous ammonia boils below room temperature when at atmospheric pressure. A storage vessel capable of 250 psi (1.7 MPa) is suitable to contain the liquid.[54] Ammonium compounds should never be allowed to come in contact with bases (unless in an intended and contained reaction), as dangerous quantities of ammonia gas could be released.Household use
Solutions of ammonia (5–10% by weight) are used as household cleaners, particularly for glass. These solutions are irritating to the eyes and mucous membranes (respiratory and digestive tracts), and to a lesser extent the skin. Caution should be used that the chemical is never mixed into any liquid containing bleach, as a poisonous gas may result. Mixing with chlorine-containing products or strong oxidants, such as household bleach, can lead to hazardous compounds such as chloramines.[55]Laboratory use of ammonia solutions
The hazards of ammonia solutions depend on the concentration: "dilute" ammonia solutions are usually 5–10% by weight (<5.62 mol/L); "concentrated" solutions are usually prepared at >25% by weight. A 25% (by weight) solution has a density of 0.907 g/cm3, and a solution that has a lower density will be more concentrated. The European Union classification of ammonia solutions is given in the table.