From Wikipedia, the free encyclopedia
| Atmosphere of Mars | |
|---|---|
| Chemical species | Mole fraction[1] |
| Carbon dioxide | 96.0% |
| Argon | 1.9% |
| Nitrogen | 1.9% |
| Oxygen | 0.145% |
| Carbon monoxide | 0.0557% |
The atmospheric pressure on the Martian surface averages 600 pascals (0.087 psi), about 0.6% of Earth's mean sea level pressure of 101.3 kilopascals (14.69 psi) and only 0.0065% that of Venus's 9.2 megapascals (1,330 psi). It ranges from a low of 30 pascals (0.0044 psi) on Olympus Mons's peak to over 1,155 pascals (0.1675 psi) in the depths of Hellas Planitia. This pressure is well below the Armstrong limit for the unprotected human body.
Mars's atmospheric mass of 25 teratonnes compares to Earth's 5148 teratonnes with a scale height of about 11 kilometres (6.8 mi) versus Earth's 7 kilometres (4.3 mi).
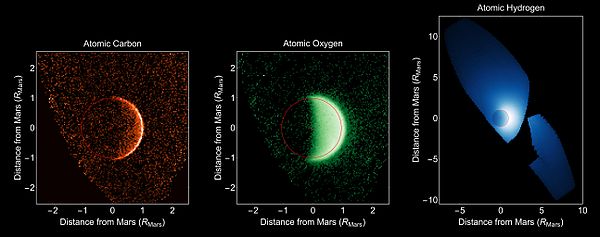
The Martian atmosphere consists of approximately 96% carbon dioxide, 1.9% argon, 1.9% nitrogen, and traces of free oxygen, carbon monoxide, water and methane, among other gases,[1] for a mean molar mass of 43.34 g/mol.[5][6] The atmosphere is quite dusty, giving the Martian sky a light brown or orange-red color when seen from the surface; data from the Mars Exploration Rovers indicate that suspended dust particles within the atmosphere are roughly 1.5 micrometres across.[7]
On 16 December 2014, NASA reported detecting an unusual increase, then decrease, in the amounts of methane in the atmosphere of the planet Mars; as well as, detecting Martian organic chemicals in powder drilled from a rock by the Curiosity rover. Also, based on deuterium to hydrogen ratio studies, much of the water at Gale Crater on Mars was found to have been lost during ancient times, before the lakebed in the crater was formed; afterwards, large amounts of water continued to be lost.[8][9][10]
Structure
| Where | Pressure |
|---|---|
| Olympus Mons summit | 0.03 kilopascals (0.0044 psi) |
| Mars average | 0.6 kilopascals (0.087 psi) |
| Hellas Planitia bottom | 1.16 kilopascals (0.168 psi) |
| Armstrong limit | 6.25 kilopascals (0.906 psi) |
| Mount Everest summit[11] | 33.7 kilopascals (4.89 psi) |
| Earth sea level | 101.3 kilopascals (14.69 psi) |
- Lower atmosphere: A warm region affected by heat from airborne dust and from the ground.
- Middle atmosphere: The region in which Mars's jetstream flows
- Upper atmosphere, or thermosphere: A region with very high temperatures, caused by heating from the Sun. Atmospheric gases start to separate from each other at these altitudes, rather than forming the even mix found in the lower atmospheric layers.
- Exosphere: Typically stated to start at 200 km (120 mi) and higher, this region is where the last wisps of atmosphere merge into the vacuum of space. There is no distinct boundary where the atmosphere ends; it just tapers away.
Observations and measurement from Earth
In 1864, William Rutter Dawes observed "that the ruddy tint of the planet does not arise from any peculiarity of its atmosphere seems to be fully proved by the fact that the redness is always deepest near the centre, where the atmosphere is thinnest."[14] Spectroscopic observations in the 1860s and 1870s[15][16] led many to think the atmosphere of Mars is similar to Earth's. In 1894, though, spectral analysis and other qualitative observations by William Wallace Campbell suggested Mars resembles the Moon, which has no appreciable atmosphere, in many respects.[15]
In 1926, photographic observations by William Hammond Wright at the Lick Observatory allowed Donald Howard Menzel to discover quantitative evidence of Mars's atmosphere.[17][18]
Composition
Carbon dioxide
The main component of the atmosphere of Mars is carbon dioxide (CO2) at 95.9%. Each pole is in continual darkness during its hemisphere's winter, and the surface gets so cold that as much as 25% of the atmospheric CO2 condenses at the polar caps into solid CO2 ice (dry ice). When the pole is again exposed to sunlight during summer, the CO2 ice sublimes back into the atmosphere. This process leads to a significant annual variation in the atmospheric pressure and atmospheric composition around the Martian poles.Argon
The atmosphere of Mars is enriched considerably with the noble gas argon, in comparison to the atmosphere of the other planets within the Solar System. Unlike carbon dioxide, the argon content of the atmosphere does not condense, and hence the total amount of argon in the Mars atmosphere is constant. However, the relative concentration at any given location can change as carbon dioxide moves in and out of the atmosphere. Recent satellite data shows an increase in atmospheric argon over the southern pole during its autumn, which dissipates the following spring.[21]
Water
Some aspects of the Martian atmosphere vary significantly. As carbon dioxide sublimes back into the atmosphere during the Martian summer, it leaves traces of water. Seasonal winds sweep off the poles at speeds approaching 400 kilometres per hour (250 mph) and transport large amounts of dust and water vapor giving rise to Earth-like frost and large cirrus clouds. These clouds of water-ice were photographed by the Opportunity rover in 2004.[22] NASA scientists working on the Phoenix Mars mission confirmed on July 31, 2008 that they had indeed found subsurface water ice at Mars's northern polar region.Methane

Trace amounts of methane (CH4), at the level of several parts per billion (ppb), were first reported in Mars's atmosphere by a team at the NASA Goddard Space Flight Center in 2003.[3][23] In March 2004, the Mars Express Orbiter and ground-based observations by three groups also suggested the presence of methane in the atmosphere with a mole fraction of about 10 ppb.[24][25][26] Large differences in the abundances were measured between observations taken in 2003 and 2006, which suggested that the methane was locally concentrated and probably seasonal.
Because methane on Mars would quickly break down due to ultraviolet radiation from the Sun and chemical reactions with other gases, its reported persistent presence in the atmosphere also necessitates the existence of a source to continually replenish the gas. Current photochemical models alone can not explain the rapid variability of the methane levels.[27][28] It had been proposed that the methane might be replenished by meteorites entering the atmosphere of Mars,[29] but researchers from Imperial College London found that the volumes of methane released this way are too low to sustain the measured levels of the gas.[30]
Research suggests that the implied methane destruction lifetime is as long as ~4 Earth years and as short as ~0.6 Earth years.[31][32] This lifetime is short enough for the atmospheric circulation to yield the observed uneven distribution of methane across the planet. In either case, the destruction lifetime for methane is much shorter than the timescale (~350 years) estimated for photochemical (UV radiation) destruction.[31] The rapid destruction (or "sink") of methane suggests that another process must dominate removal of atmospheric methane on Mars, and it must be more efficient than destruction by light by a factor of 100 to 600.[32][31] This unexplained fast destruction rate also suggests a very active replenishing source.[33] A possibility is that the methane is not consumed at all, but rather condenses and evaporates seasonally from clathrates.[34] Another possibility is that methane reacts with tumbling surface sand quartz (SiO
2) and olivine to form covalent Si–CH
3 bonds.[35]
Although the methane could stem from a geological source, the lack of current volcanism, hydrothermal activity or hotspots are not favorable for a geological explanation. Living microorganisms, such as methanogens, are another possible source, but no evidence exists for the presence of such organisms anywhere on Mars. Roscosmos and ESA are planning to look for companion gases that may suggest which sources are most likely.[36][37] In the Earth's oceans, biological methane production tends to be accompanied by ethane, whereas volcanic methane is accompanied by sulfur dioxide.[37] Several studies of trace gases in the Martian atmosphere have found no evidence for sulfur dioxide in the Martian atmosphere, which makes volcanism unlikely to be the source of methane.[38][39]
The principal candidates for the origin of Mars methane include non-biological processes such as water–rock reactions, radiolysis of water, and pyrite formation, all of which produce H2 that could then generate methane and other hydrocarbons via Fischer–Tropsch synthesis with CO and CO2.[40] It has also been shown that methane could be produced by a process involving water, carbon dioxide, and the mineral olivine, which is known to be common on Mars.[41] The required conditions for this reaction (i.e. high temperature and pressure) do not exist on the surface, but may exist within the crust.[42] A detection of the mineral by-product serpentinite would suggest that this process is occurring. An analog on Earth suggests that low-temperature production and exhalation of methane from serpentinized rocks may be possible on Mars.[43] Another possible geophysical source could be clathrate hydrates.[44]
A group of Mexican scientists performed plasma experiments in a synthetic Mars atmosphere and found that bursts of methane can be produced when a discharge interacts with water ice. A potential source of the discharges can be the electrification of dust particles from sand storms and dust devils. The ice can be found in trenches or in the permafrost. The electrical discharge ionizes gaseous CO2 and water molecules and their byproducts recombine to produce methane. The results obtained show that pulsed electrical discharges over ice samples in a Martian atmosphere produce about 1.41×1016 molecules of methane per joule of applied energy.[45][46]
In contrast to the findings described above, studies by Kevin Zahnle, a planetary scientist at NASA's Ames Research Center, and two colleagues concluded that "there is as yet no compelling evidence for methane on Mars". They argued that the strongest reported observations of the gas to date have been taken at frequencies where interference from methane in Earth's atmosphere is particularly difficult to remove, and are thus unreliable. Additionally, they claimed that the published observations most favorable to interpretation as indicative of Martian methane are also consistent with no methane being present on Mars.[47][48][49]
In 2011, NASA scientists reported a comprehensive search using ground-based high-resolution infrared spectroscopy for trace species (including methane) on Mars, deriving sensitive upper limits for methane (<7 ppbv), ethane (<0.2 ppbv), methanol (<19 and="" others="" ppbv="" sub="">2
In August 2012, the Curiosity rover landed on Mars. The rover's instruments are capable of making precise abundance measurements that also distinguish between different isotopologues of methane.[52] Efforts to identify the sources of terrestrial methane have found that measurements of different methane isotopologues do not necessarily distinguish between possible geologic and biogenic sources, but the abundances of other cogenerated gases, such as ethane (C2H6), relative to methane do: the ethane–methane abundance ratio is < 0.001 for biogenic sources, whereas other sources produce nearly equivalent amounts of methane and ethane.[53]
The first measurements with Curiosity's Tunable Laser Spectrometer (TLS) indicated that there were less than 5 ppb of methane at the landing site.[54][55][56][57] On 19 July 2013, NASA scientists published the results of a new analysis of the atmosphere of Mars, reporting a lack of methane around the landing site of the Curiosity rover. In addition, the scientists found evidence that Mars "has lost a good deal of its atmosphere over time", based on the abundance of isotopic compositions of gases, particularly those related to argon and carbon.[58][59][60] On 19 September 2013, NASA scientists used further measurements from Curiosity to report a non-detection of atmospheric methane with a measured value of 0.18±0.67 ppbv corresponding to an upper limit of only 1.3 ppbv (95% confidence limit). As a result, they concluded that current methanogenic microbial activity on Mars is extremely unlikely.[61][62][63]
On 16 December 2014, NASA reported that Curiosity had detected a tenfold increase ('spike') in methane in the atmosphere around it in late 2013 and early 2014. Four measurements taken over two months in this period averaged 7 ppb. Before and after that, readings averaged around one-tenth that level.[8][9][10]
The Indian Mars Orbiter Mission, which entered orbit around Mars on 24 September 2014, is equipped with a Fabry–Pérot interferometer to measure atmospheric methane at a level of several ppb.[64] The ExoMars Trace Gas Orbiter planned to launch in 2016 would further study the methane, as well as its decomposition products such as formaldehyde and methanol.[65][66]
Sulfur dioxide
Sulfur dioxide in the atmosphere is thought to be a tracer of current volcanic activity. It has become especially interesting due to the long-standing controversy of methane on Mars. If methane on Mars were being produced by volcanoes (as it is in part on Earth) we would expect to find sulfur dioxide in large quantities. Several teams have searched for sulfur dioxide on Mars using the NASA Infrared Telescope Facility. No sulfur dioxide was detected in these studies, but they were able to place stringent upper limits on the atmospheric concentration of 0.2 ppb.[38][39] In March 2013, a team led by scientists at NASA Goddard Space Flight Center reported a detection of SO2 in Rocknest (Mars) soil samples analyzed by the Curiosity rover.[67]Ozone
As reported by the European Space Agency (ESA) on September 29, 2013, a new comparison of spacecraft data with computer models explains how global atmospheric circulation creates a layer of ozone above Mars's southern pole in winter. Ozone was most likely difficult to detect on Mars because its concentration is typically 300 times lower than on Earth, although it varies greatly with location and time. In recent years, the SPICAM UV spectrometer on board Mars Express has shown the presence of two distinct ozone layers at low-to-mid latitudes. These comprise a persistent, near-surface layer below an altitude of 30 km, a separate layer that is only present in northern spring and summer with an altitude varying from 30 to 60 km, and another separate layer that exists 40–60 km above the southern pole in winter, with no counterpart above the Mars's north pole. This third ozone layer shows an abrupt decrease in elevation between 75 and 50 degrees south. SPICAM detected a gradual increase in ozone concentration at 50 km until midwinter, after which it slowly decreased to very low concentrations, with no layer detectable above 35 km. The authors of the paper in Nature Geoscience think that the observed polar ozone layers are the result of the same atmospheric circulation pattern that creates a distinct oxygen emission recently identified in the polar night and also present in Earth's atmosphere. This circulation takes the form of a huge Hadley cell in which warmer air rises and travels poleward before cooling and sinking at higher latitudes. Mars is on a quite elliptical orbit and has a large axial tilt, which causes extreme seasonal variations in temperature amongst the northern and southern hemispheres. Mars's temperature difference greatly influences the amount of water vapor in the atmosphere, because warmer air can contain more moisture. This, in turn, affects the production of ozone-destroying hydrogen radicals.
Potential for use by humans
The atmosphere of Mars is a resource of known composition available at any landing site on Mars. It has been proposed that human exploration of Mars could use carbon dioxide (CO2) from the Martian atmosphere to make rocket fuel for the return mission. Mission studies that propose using the atmosphere in this way include the Mars Direct proposal of Robert Zubrin and the NASA Design reference mission study. Two major chemical pathways for use of the carbon dioxide are the Sabatier reaction, converting atmospheric carbon dioxide along with additional hydrogen (H2), to produce methane (CH4) and oxygen (O2), and electrolysis, using a zirconia solid oxide electrolyte to split the carbon dioxide into oxygen (O2) and carbon monoxide (CO).History
Mars's atmosphere is thought to have changed over the course of the planet's lifetime, with evidence suggesting the possibility that Mars had large oceans a few billion years ago.[68] As stated in the Mars ocean hypothesis, atmospheric pressure on the present-day Martian surface only exceeds that of the triple point of water (6.11 hectopascals (0.0886 psi)) in the lowest elevations; at higher elevations water can exist only in solid or vapor form. Annual mean temperatures at the surface are currently less than 210 K (−63 °C; −82 °F), significantly lower than that needed to sustain liquid water.However, early in its history Mars may have had conditions more conducive to retaining liquid water at the surface. In 2013, scientists published that Mars might have had an "oxygen-rich" atmosphere billions of years ago.[69][70]
Possible causes for the depletion of a previously thicker Martian atmosphere include:
- Gradual erosion of the atmosphere by solar wind,[71] possibly helped by Mars's magnetic-field irregularities;[72]
- Catastrophic collision by a body large enough to blow away a significant percentage of the atmosphere;[72]
- Mars's low gravity allowing the atmosphere to "blow off" into space by Jeans escape.[73]
Images
-
Mars Pathfinder – Martian sky with water ice clouds.
Martian sunset by Spirit rover at Gusev crater (May, 2005).
Martian sunset by Pathfinder at Ares Vallis (July, 1997).