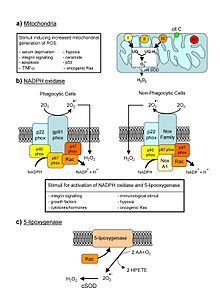
Major cellular sources of ROS in living non-photosynthetic cells. From a review by Novo and Parola, 2008.
Reactive oxygen species (ROS) are chemically reactive chemical species containing oxygen. Examples include peroxides, superoxide, hydroxyl radical, singlet oxygen, and alpha-oxygen.
In a biological context, ROS are formed as a natural byproduct of the normal metabolism of oxygen and have important roles in cell signaling and homeostasis. However, during times of environmental stress (e.g., UV or heat exposure), ROS levels can increase dramatically. This may result in significant damage to cell structures. Cumulatively, this is known as oxidative stress.
The production of ROS is strongly influenced by stress factor responses
in plants, these factors that increase ROS production include drought,
salinity, chilling, nutrient deficiency, metal toxicity and UV-B radiation. ROS are also generated by exogenous sources such as ionizing radiation.
Formation and decomposition
The reduction of molecular oxygen (O2) produces superoxide (•O−
2) and is the precursor of most other reactive oxygen species:
2) and is the precursor of most other reactive oxygen species:
- O2 + e− → •O−
2
Dismutation of superoxide produces hydrogen peroxide (H2O2):
- 2 H+ + •O−
2 + •O−
2 → H2O2 + O2
Hydrogen peroxide in turn may be partially reduced to hydroxyl radical (•OH) or fully reduced to water:
- H2O2 + e− → HO− + •OH
- 2 H+ + 2 e− + H2O2 → 2 H2O
Exogenous ROS
Exogenous ROS can be produced from pollutants, tobacco, smoke, drugs, xenobiotics, or radiation.
Ionizing radiation can generate damaging intermediates through the interaction with water, a process termed radiolysis.
Since water comprises 55–60% of the human body, the probability of
radiolysis is quite high under the presence of ionizing radiation. In
the process, water loses an electron and becomes highly reactive. Then
through a three-step chain reaction, water is sequentially converted to hydroxyl radical (•OH), hydrogen peroxide (H2O2), superoxide radical (•O−
2), and ultimately oxygen (O2).
2), and ultimately oxygen (O2).
The hydroxyl radical is extremely reactive and immediately
removes electrons from any molecule in its path, turning that molecule
into a free radical and thus propagating a chain reaction. However,
hydrogen peroxide is actually more damaging to DNA than the hydroxyl
radical, since the lower reactivity of hydrogen peroxide provides enough
time for the molecule to travel into the nucleus of the cell,
subsequently reacting with macromolecules such as DNA.
Endogenous ROS
ROS
are produced intracellularly through multiple mechanisms and depending
on the cell and tissue types, the major sources being the "professional"
producers of ROS: NADPH oxidase (NOX) complexes (7 distinct isoforms) in cell membranes, mitochondria, peroxisomes, and endoplasmic reticulum. Mitochondria convert energy for the cell into a usable form, adenosine triphosphate (ATP). The process in which ATP is produced, called oxidative phosphorylation, involves the transport of protons (hydrogen ions) across the inner mitochondrial membrane by means of the electron transport chain. In the electron transport chain, electrons are passed through a series of proteins
via oxidation-reduction reactions, with each acceptor protein along the
chain having a greater reduction potential than the previous. The last
destination for an electron along this chain is an oxygen molecule. In
normal conditions, the oxygen is reduced to produce water; however, in
about 0.1–2% of electrons passing through the chain (this number derives
from studies in isolated mitochondria, though the exact rate in live
organisms is yet to be fully agreed upon), oxygen is instead prematurely
and incompletely reduced to give the superoxide radical (•O−
2), most well documented for Complex I and Complex III. Superoxide is not particularly reactive by itself, but can inactivate specific enzymes or initiate lipid peroxidation in its protonated form, hydroperoxyl HO•
2. The pKa of hydroperoxyl is 4.8. Thus, at physiological pH, the majority will exist as superoxide anion.
2), most well documented for Complex I and Complex III. Superoxide is not particularly reactive by itself, but can inactivate specific enzymes or initiate lipid peroxidation in its protonated form, hydroperoxyl HO•
2. The pKa of hydroperoxyl is 4.8. Thus, at physiological pH, the majority will exist as superoxide anion.
If too much damage is present in mitochondria, a cell undergoes apoptosis or programmed cell death.
Bcl-2 proteins are layered on the surface of the mitochondria, detect
damage, and activate a class of proteins called Bax, which punch holes
in the mitochondrial membrane, causing cytochrome C to leak out. This
cytochrome C binds to Apaf-1, or apoptotic protease activating factor-1,
which is free-floating in the cell's cytoplasm. Using energy from the
ATPs in the mitochondrion, the Apaf-1 and cytochrome C bind together to
form apoptosomes. The apoptosomes
bind to and activate caspase-9, another free-floating protein. The
caspase-9 then cleaves the proteins of the mitochondrial membrane,
causing it to break down and start a chain reaction of protein
denaturation and eventually phagocytosis of the cell.
Superoxide dismutase
Superoxide dismutases
(SOD) are a class of enzymes that catalyze the dismutation of
superoxide into oxygen and hydrogen peroxide. As such, they are an
important antioxidant
defense in nearly all cells exposed to oxygen. In mammals and most
chordates, three forms of superoxide dismutase are present. SOD1 is
located primarily in the cytoplasm, SOD2 in the mitochondria and SOD3 is
extracellular. The first is a dimer (consists of two units), while the
others are tetramers (four subunits). SOD1 and SOD3 contain copper and
zinc ions, while SOD2 has a manganese ion in its reactive centre. The
genes are located on chromosomes 21, 6, and 4, respectively (21q22.1,
6q25.3 and 4p15.3-p15.1).
The SOD-catalysed dismutation of superoxide may be written with the following half-reactions:
- M(n+1)+ − SOD + O−
2 → Mn+ − SOD + O2 - Mn+ − SOD + O−
2 + 2H+ → M(n+1)+ − SOD + H2O2.
where M = Cu (n = 1); Mn (n = 2); Fe (n = 2); Ni (n = 2). In this reaction the oxidation state of the metal cation oscillates between n and n + 1.
Catalase, which is concentrated in peroxisomes located next to mitochondria, reacts with the hydrogen peroxide to catalyze the formation of water and oxygen. Glutathione peroxidase
reduces hydrogen peroxide by transferring the energy of the reactive
peroxides to a very small sulfur-containing protein called glutathione.
The sulfur contained in these enzymes acts as the reactive center,
carrying reactive electrons from the peroxide to the glutathione. Peroxiredoxins also degrade H2O2, within the mitochondria, cytosol, and nucleus.
- 2 H2O2 → 2 H2O + O2 (catalase)
- 2GSH + H2O2 → GS–SG + 2H2O (glutathione peroxidase)
Singlet oxygen
Another type of reactive oxygen species is singlet oxygen (1O2) which is produced for example as byproduct of photosynthesis in plants. In the presence of light and oxygen, photosensitizers such as chlorophyll may convert triplet (3O2) to singlet oxygen:
Singlet oxygen is highly reactive, especially with organic compounds
that contain double bonds. The resulting damage caused by singlet oxygen
reduces the photosynthetic efficiency of chloroplasts. In plants exposed to excess light, the increased production of singlet oxygen can result in cell death. Various substances such as carotenoids, tocopherols and plastoquinones
contained in chloroplasts quench singlet oxygen and protect against its
toxic effects. In addition to direct toxicity, singlet oxygen acts a signaling molecule. Oxidized products of β-carotene arising from the presence of singlet oxygen act as second messengers that can either protect against singlet oxygen induced toxicity or initiate programmed cell death. Levels of jasmonate
play a key role in the decision between cell acclimation or cell death
in response to elevated levels of this reactive oxygen species.
Damaging effects
Effects of ROS on cell metabolism are well documented in a variety of species. These include not only roles in apoptosis (programmed cell death) but also positive effects such as the induction of host defense genes and mobilization of ion transport systems. This implicates them in control of cellular function. In particular, platelets involved in wound repair and blood homeostasis release ROS to recruit additional platelets to sites of injury. These also provide a link to the adaptive immune system via the recruitment of leukocytes.
Reactive oxygen species are implicated in cellular activity to a variety of inflammatory responses including cardiovascular disease. They may also be involved in hearing impairment via cochlear damage induced by elevated sound levels, in ototoxicity of drugs such as cisplatin, and in congenital deafness in both animals and humans. ROS are also implicated in mediation of apoptosis or programmed cell death and ischaemic injury. Specific examples include stroke and heart attack.
In general, harmful effects of reactive oxygen species on the cell are most often:
- damage of DNA or RNA
- oxidations of polyunsaturated fatty acids in lipids (lipid peroxidation)
- oxidations of amino acids in proteins
- oxidative deactivation of specific enzymes by oxidation of co-factors
Pathogen response
When a plant recognizes an attacking pathogen, one of the first induced reactions is to rapidly produce superoxide (O−
2) or hydrogen peroxide (H
2O
2) to strengthen the cell wall. This prevents the spread of the pathogen to other parts of the plant, essentially forming a net around the pathogen to restrict movement and reproduction.
2) or hydrogen peroxide (H
2O
2) to strengthen the cell wall. This prevents the spread of the pathogen to other parts of the plant, essentially forming a net around the pathogen to restrict movement and reproduction.
In the mammalian host, ROS is induced as an antimicrobial
defense. To highlight the importance of this defense, individuals with
chronic granulomatous disease who have deficiencies in generating ROS,
are highly susceptible to infection by a broad range of microbes
including Salmonella enterica, Staphylococcus aureus, Serratia marcescens, and Aspergillus spp.
The exact manner in which ROS defends the host from invading
microbe is not fully understood. One of the more likely modes of defense
is damage to microbial DNA. Studies using Salmonella demonstrated that
DNA repair mechanisms were required to resist killing by ROS. More
recently, a role for ROS in antiviral defense mechanisms has been
demonstrated via Rig-like helicase-1 and mitochondrial antiviral
signaling protein. Increased levels of ROS potentiate signaling through
this mitochondria-associated antiviral receptor to activate interferon
regulatory factor (IRF)-3, IRF-7, and nuclear factor kappa B (NF-κB),
resulting in an antiviral state.
Respiratory epithelial cells were recently demonstrated to induce
mitrochondrial ROS in response to influenza infection. This induction of
ROS led to the induction of type III interferon and the induction of an
antiviral state, limiting viral replication.
In host defense against mycobacteria, ROS play a role, although direct
killing is likely not the key mechanism; rather, ROS likely affect
ROS-dependent signalling controls, such as cytokine production,
autophagy, and granuloma formation.
Oxidative damage
In aerobic organisms the energy needed to fuel biological functions is produced in the mitochondria via the electron transport chain. In addition to energy, reactive oxygen species (ROS) with the potential to cause cellular damage are produced. ROS can damage lipid, DNA, RNA, and proteins, which, in theory, contributes to the physiology of aging.
ROS are produced as a normal product of cellular metabolism. In particular, one major contributor to oxidative damage is hydrogen peroxide (H2O2), which is converted from superoxide that leaks from the mitochondria. Catalase and superoxide dismutase ameliorate the damaging effects of hydrogen peroxide and superoxide, respectively, by converting these compounds into oxygen and hydrogen peroxide (which is later converted to water), resulting in the production of benign molecules.
However, this conversion is not 100% efficient, and residual peroxides
persist in the cell. While ROS are produced as a product of normal
cellular functioning, excessive amounts can cause deleterious effects.
Memory capabilities decline with age, evident in human degenerative diseases such as Alzheimer's disease,
which is accompanied by an accumulation of oxidative damage. Current
studies demonstrate that the accumulation of ROS can decrease an
organism's fitness
because oxidative damage is a contributor to senescence. In particular,
the accumulation of oxidative damage may lead to cognitive dysfunction,
as demonstrated in a study in which old rats were given mitochondrial metabolites and then given cognitive tests. Results showed that the rats
performed better after receiving the metabolites, suggesting that the
metabolites reduced oxidative damage and improved mitochondrial
function. Accumulating oxidative damage can then affect the efficiency of mitochondria and further increase the rate of ROS production. The accumulation of oxidative damage and its implications for aging depends on the particular tissue
type where the damage is occurring. Additional experimental results
suggest that oxidative damage is responsible for age-related decline in brain functioning. Older gerbils were found to have higher levels of oxidized protein in comparison to younger gerbils. Treatment of old and young mice with a spin trapping
compound caused a decrease in the level of oxidized proteins in older
gerbils but did not have an effect on younger gerbils. In addition,
older gerbils performed cognitive tasks better during treatment but
ceased functional capacity when treatment was discontinued, causing
oxidized protein levels to increase. This led researchers to conclude
that oxidation of cellular proteins is potentially important for brain
function.
Cause of aging
According to the free radical theory of aging,
oxidative damage initiated by reactive oxygen species is a major
contributor to the functional decline that is characteristic of aging.
While studies in invertebrate models indicate that animals genetically
engineered to lack specific antioxidant enzymes (such as SOD), in
general, show a shortened lifespan (as one would expect from the
theory), the converse manipulation, increasing the levels of antioxidant
enzymes, has yielded inconsistent effects on lifespan (though some
studies in Drosophila
do show that lifespan can be increased by the overexpression of MnSOD
or glutathione biosynthesizing enzymes). Also contrary to this theory,
deletion of mitochondrial SOD2 can extend lifespan in Caenorhabditis elegans.
In mice, the story is somewhat similar. Deleting antioxidant
enzymes, in general, yields shorter lifespan, though overexpression
studies have not (with some recent exceptions) consistently extended
lifespan. Study of a rat model of premature aging found increased oxidative stress, reduced antioxidant enzyme activity and substantially greater DNA damage in the brain neocortex and hippocampus of the prematurely aged rats than in normally aging control rats. The DNA damage 8-OHdG is a product of ROS interaction with DNA. Numerous studies have shown that 8-OHdG increases in different mammalian organs with age.
Male infertility
Exposure of spermatozoa to oxidative stress is a major causative agent of male infertility. Sperm DNA fragmentation, caused by oxidative stress, appears to be an important factor in the etiology of male infertility. A high level of the oxidative DNA damage 8-OHdG is associated with abnormal spermatozoa and male infertility.
Cancer
ROS are constantly generated and eliminated in the biological system and are required to drive regulatory pathways.
Under normal physiological conditions, cells control ROS levels by
balancing the generation of ROS with their elimination by scavenging
system. But under oxidative stress conditions, excessive ROS can damage
cellular proteins, lipids and DNA, leading to fatal lesions in cell that
contribute to carcinogenesis.
Cancer cells exhibit greater ROS stress than normal cells do,
partly due to oncogenic stimulation, increased metabolic activity and
mitochondrial malfunction. ROS is a double-edged sword. On one hand, at
low levels, ROS facilitates cancer cell survival since cell-cycle
progression driven by growth factors and receptor tyrosine kinases (RTK)
require ROS for activation
and chronic inflammation, a major mediator of cancer, is regulated by
ROS. On the other hand, a high level of ROS can suppress tumor growth
through the sustained activation of cell-cycle inhibitor
and induction of cell death as well as senescence by damaging
macromolecules. In fact, most of the chemotherapeutic and
radiotherapeutic agents kill cancer cells by augmenting ROS stress.
The ability of cancer cells to distinguish between ROS as a survival or
apoptotic signal is controlled by the dosage, duration, type, and site
of ROS production. Modest levels of ROS are required for cancer cells to
survive, whereas excessive levels kill them.
Metabolic adaptation in tumors balances the cells' need for
energy with equally important need for macromolecular building blocks
and tighter control of redox balance. As a result, production of NADPH
is greatly enhanced, which functions as a cofactor to provide reducing
power in many enzymatic reactions for macromolecular biosynthesis and at
the same time rescuing the cells from excessive ROS produced during
rapid proliferation. Cells counterbalance the detrimental effects of ROS
by producing antioxidant molecules, such as reduced glutathione (GSH)
and thioredoxin (TRX), which rely on the reducing power of NADPH to
maintain their activities.
Most risk factors associated with cancer interact with cells
through the generation of ROS. ROS then activate various transcription
factors such as nuclear factor kappa-light-chain-enhancer of activated B
cells (NF-κB), activator protein-1 (AP-1), hypoxia-inducible factor-1α
and signal transducer and activator of transcription 3 (STAT3), leading
to expression of proteins that control inflammation; cellular
transformation; tumor cell survival; tumor cell proliferation; and
invasion, agiogenesis as well as metastasis. And ROS also control the
expression of various tumor suppressor genes such as p53, retinoblastoma
gene (Rb), and phosphatase and tensin homolog (PTEN).
Carcinogenesis
ROS-related
oxidation of DNA is one of the main causes of mutations, which can
produce several types of DNA damage, including non-bulky (8-oxoguanine
and formamidopyrimidine) and bulky (cyclopurine and etheno adducts) base
modifications, abasic sites, non-conventional single-strand breaks,
protein-DNA adducts, and intra/interstrand DNA crosslinks.
It has been estimated that endogenous ROS produced via normal cell
metabolism modify approximately 20,000 bases of DNA per day in a single
cell. 8-oxoguanine is the most abundant among various oxidized
nitrogeneous bases observed. During DNA replication, DNA polymerase
mispairs 8-oxoguanine with adenine, leading to a G→T transversion
mutation. The resulting genomic instability directly contributes to
carcinogenesis. Cellular transformation leads to cancer and interaction
of atypical PKC-ζ isoform with p47phox controls ROS production and
transformation from apoptotic cancer stem cells through blebbishield emergency program.
Cell proliferation
Uncontrolled
proliferation is a hallmark of cancer cells. Both exogenous and
endogenous ROS have been shown to enhance proliferation of cancer cells.
The role of ROS in promoting tumor proliferation is further supported
by the observation that agents with potential to inhibit ROS generation
can also inhibit cancer cell proliferation.
Although ROS can promote tumor cell proliferation, a great increase in
ROS has been associated with reduced cancer cell proliferation by
induction of G2/M cell cycle arrest; increased phosphorylation of ataxia
telangiectasia mutated (ATM), checkpoint kinase 1 (Chk 1), Chk 2; and
reduced cell division cycle 25 homolog c (CDC25).
Cell death
A cancer cell can die in three ways: apoptosis, necrosis, and autophagy. Excessive ROS can induce apoptosis through both the extrinsic and intrinsic pathways.
In the extrinsic pathway of apoptosis, ROS are generated by Fas ligand
as an upstream event for Fas activation via phosphorylation, which is
necessary for subsequent recruitment of Fas-associated protein with
death domain and caspase 8 as well as apoptosis induction.
In the intrinsic pathway, ROS function to facilitate cytochrome c
release by activating pore-stabilizing proteins (Bcl-2 and Bcl-xL) as
well as inhibiting pore-destabilizing proteins (Bcl-2-associated X
protein, Bcl-2 homologous antagonist/killer).
The intrinsic pathway is also known as the caspase cascade and is
induced through mitochondrial damage which triggers the release of
cytochrome c. DNA damage, oxidative stress, and loss of mitochondrial
membrane potential lead to the release of the pro-apoptotic proteins
mentioned above stimulating apoptosis.
Mitochondrial damage is closely linked to apoptosis and since
mitochondria are easily targeted there is potential for cancer therapy.
The cytotoxic nature of ROS is a driving force behind apoptosis,
but in even higher amounts, ROS can result in both apoptosis and
necrosis, a form of uncontrolled cell death, in cancer cells.
Numerous studies have shown the pathways and associations between
ROS levels and apoptosis, but a newer line of study has connected ROS
levels and autophagy.
ROS can also induce cell death through autophagy, which is a
self-catabolic process involving sequestration of cytoplasmic contents
(exhausted or damaged organelles and protein aggregates) for degradation
in lysosomes.
Therefore, autophagy can also regulate the cell’s health in times of
oxidative stress. Autophagy can be induced by ROS levels through many
different pathways in the cell in an attempt to dispose of harmful
organelles and prevent damage, such as carcinogens, without inducing
apoptosis.
Autophagic cell death can be prompted by the over expression of
autophagy where the cell digests too much of itself in an attempt to
minimize the damage and can no longer survive. When this type of cell
death occurs, an increase or loss of control of autophagy regulating
genes is commonly co-observed.
Thus, once a more in-depth understanding of autophagic cell death is
attained and its relation to ROS, this form of programmed cell death may
serve as a future cancer therapy.
Autophagy and apoptosis are two different cell death mechanisms brought
on by high levels of ROS in the cells, however; autophagy and apoptosis
rarely act through strictly independent pathways. There is a clear
connection between ROS and autophagy and a correlation seen between
excessive amounts of ROS leading to apoptosis.
The depolarization of the mitochondrial membrane is also characteristic
of the initiation of autophagy. When mitochondria are damaged and begin
to release ROS, autophagy is initiated to dispose of the damaging
organelle. If a drug targets mitochondria and creates ROS, autophagy may
dispose of so many mitochondria and other damaged organelles that the
cell is no longer viable. The extensive amount of ROS and mitochondrial
damage may also signal for apoptosis. The balance of autophagy within
the cell and the crosstalk between autophagy and apoptosis mediated by
ROS is crucial for a cell’s survival. This crosstalk and connection
between autophagy and apoptosis could be a mechanism targeted by cancer
therapies or used in combination therapies for highly resistant cancers.
Tumor cell invasion, angiogenesis and metastasis
After
growth factor stimulation of RTKs, ROS can trigger activation of
signaling pathways involved in cell migration and invasion such as
members of the mitogen activated protein kinase (MAPK) family –
extracellular regulated kinase (ERK), c-jun NH-2 terminal kinase (JNK)
and p38 MAPK. ROS can also promote migration by augmenting
phosphorylation of the focal adhesion kinase (FAK) p130Cas and paxilin.
Both in vitro and in vivo, ROS have been shown to induce
transcription factors and modulate signaling molecules involved in
angiogenesis (MMP, VEGF) and metastasis (upregulation of AP-1, CXCR4,
AKT and downregulation of PTEN).
Chronic inflammation and cancer
Experimental
and epidemiologic research over the past several years has indicated
close associations among ROS, chronic inflammation, and cancer.
ROS induces chronic inflammation by the induction of COX-2,
inflammatory cytokines (TNFα, interleukin 1 (IL-1), IL-6), chemokines
(IL-8, CXCR4) and pro-inflammatory transcription factors (NF-κB). These chemokines and chemokine receptors, in turn, promote invasion and metastasis of various tumor types.
Cancer therapy
Both
ROS-elevating and ROS-eliminating strategies have been developed with
the former being predominantly used. Cancer cells with elevated ROS
levels depend heavily on the antioxidant defense system. ROS-elevating
drugs further increase cellular ROS stress level, either by direct
ROS-generation (e.g. motexafin gadolinium, elesclomol) or by agents that
abrogate the inherent antioxidant system such as SOD inhibitor (e.g.
ATN-224, 2-methoxyestradiol) and GSH inhibitor (e.g. PEITC, buthionine
sulfoximine (BSO)). The result is an overall increase in endogenous ROS,
which when above a cellular tolerability threshold, may induce cell
death.
On the other hand, normal cells appear to have, under lower basal
stress and reserve, a higher capacity to cope with additional
ROS-generating insults than cancer cells do. Therefore, the elevation of ROS in all cells can be used to achieve the selective killing of cancer cells.
Radiotherapy also relies on ROS toxicity to eradicate tumor
cells. Radiotherapy uses X-rays, γ-rays as well as heavy particle
radiation such as protons and neutrons to induce ROS-mediated cell death
and mitotic failure.
Due to the dual role of ROS, both prooxidant and
antioxidant-based anticancer agents have been developed. However,
modulation of ROS signaling alone seems not to be an ideal approach due
to adaptation of cancer cells to ROS stress, redundant pathways for
supporting cancer growth and toxicity from ROS-generating anticancer
drugs. Combinations of ROS-generating drugs with pharmaceuticals that
can break the redox adaptation could be a better strategy for enhancing
cancer cell cytotoxicity.
James Watson and others
have proposed that lack of intracellular ROS due to a lack of physical
exercise may contribute to the malignant progression of cancer, because
spikes of ROS are needed to correctly fold proteins in the endoplasmatic
reticulum and low ROS levels may thus aspecifically hamper the
formation of tumor suppressor proteins.
Since physical exercise induces temporary spikes of ROS, this may
explain why physical exercise is beneficial for cancer patient
prognosis.
Moreover, high inducers of ROS such as 2-deoxy-D-glucose and
carbohydrate-based inducers of cellular stress induce cancer cell death
more potently because they exploit cancer cell high avidity for sugars.
Positive role of ROS in memory
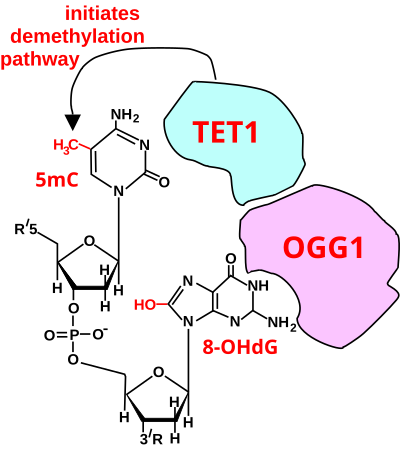
Initiation of DNA demethylation at a CpG site. In adult somatic cells DNA methylation typically occurs in the context of CpG dinucleotides (CpG sites), forming 5-methylcytosine-pG, or 5mCpG. Reactive oxygen species (ROS) may attack guanine at the dinucleotide site, forming 8-hydroxy-2'-deoxyguanosine (8-OHdG), and resulting in a 5mCp-8-OHdG dinucleotide site. The base excision repair enzyme OGG1 targets 8-OHdG and binds to the lesion without immediate excision. OGG1, present at a 5mCp-8-OHdG site recruits TET1 and TET1 oxidizes the 5mC adjacent to the 8-OHdG. This initiates demethylation of 5mC.
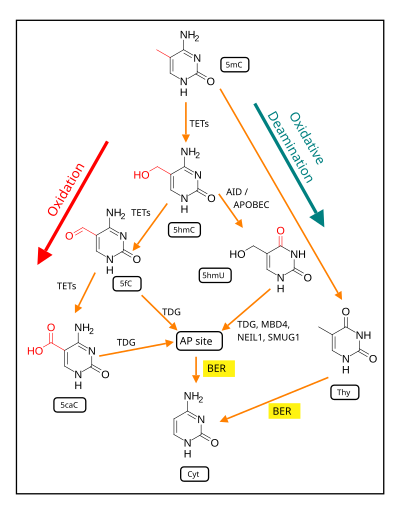
Demethylation of 5-Methylcytosine (5mC) in neuron DNA. As reviewed in 2018, in brain neurons, 5mC is oxidized by the ten-eleven translocation (TET) family of dioxygenases (TET1, TET2, TET3) to generate 5-hydroxymethylcytosine
(5hmC). In successive steps TET enzymes further hydroxylate 5hmC to
generate 5-formylcytosine (5fC) and 5-carboxylcytosine (5caC). Thymine-DNA glycosylase (TDG) recognizes the intermediate bases 5fC and 5caC and excises the glycosidic bond resulting in an apyrimidinic site (AP site).
In an alternative oxidative deamination pathway, 5hmC can be
oxidatively deaminated by activity-induced cytidine
deaminase/apolipoprotein B mRNA editing complex (AID/APOBEC) deaminases to form 5-hydroxymethyluracil (5hmU) or 5mC can be converted to thymine (Thy). 5hmU can be cleaved by TDG, single-strand-selective monofunctional uracil-DNA glycosylase 1 (SMUG1), Nei-Like DNA Glycosylase 1 (NEIL1), or methyl-CpG binding protein 4 (MBD4). AP sites and T:G mismatches are then repaired by base excision repair (BER) enzymes to yield cytosine (Cyt).
Two reviews summarize the large body of evidence, reported largely between 1996 and 2011, for the critical and essential role of ROS in memory formation. A recent additional body of evidence indicates that both the formation and storage of memory depend on epigenetic modifications in neurons, including alterations in neuronal DNA methylation. The two bodies of information on memory formation appear to be connected in 2016 by the work of Zhou et al, who showed that ROS have a central role in epigenetic DNA demethylation.
In mammalian nuclear DNA, a methyl group can be added, by a DNA methyltransferase,
to the 5th carbon of cytosine to form 5mC (see red methyl group added
to form 5mC near the top of the first figure). The DNA
methyltransferases most often form 5mC within the dinucleotide sequence
"cytosine-phosphate-guanine" to form 5mCpG. This addition is a major
type of epigenetic alteration and it can silence gene expression. Methylated cytosine can also be demethylated, an epigenetic alteration that can increase the expression of a gene. A major enzyme involved in demethylating 5mCpG is TET1. However, TET1 is only able to act on 5mCpG if an ROS has first acted on the guanine to form 8-hydroxy-2'-deoxyguanosine (8-OHdG), resulting in a 5mCp-8-OHdG dinucleotide (see first figure). However, TET1 is only able to act on the 5mC part of the dinucleotide when the base excision repair enzyme OGG1 binds to the 8-OHdG lesion without immediate excision. Adherence of OGG1 to the 5mCp-8-OHdG site recruits TET1
and TET1 then oxidizes the 5mC adjacent to 8-OHdG, as shown in the
first figure, initiating a demethylation pathway shown in the second
figure.
In 2016 Halder et al. using mice, and in 2017 Duke et al. using rats, subjected the rodents to contextual fear conditioning, causing an especially strong long-term memory to form. At 24 hours after the conditioning, in the hippocampus of rats, the expression of 1,048 genes was down-regulated (usually associated with hypermethylated gene promoters)
and the expression of 564 genes was up-regulated (often associated with
hypomethylated gene promoters). At 24 hours after training, 9.2% of
the genes in the rat genome of hippocampus
neurons were differentially methylated. However while the hippocampus
is essential for learning new information it does not store information
itself. In the mouse experiments of Halder, 1,206 differentially
methylated genes were seen in the hippocampus one hour after contextual
fear conditioning but these were reversed and not seen after four weeks.
In contrast with the absence of long-term methylation changes in the
hippocampus, substantial differential methylation could be detected in cortical
neurons during memory maintenance. There were 1,223 differentially
methylated genes in the anterior cingulate cortex of mice four weeks
after contextual fear conditioning.
The thousands of CpG sites being demethylated during memory
formation depend on ROS in an initial step. The altered protein
expression in neurons, controlled in part by ROS-dependent demethylation
of CpG sites in gene promoters within neuron DNA, are central to memory
formation.

![{\displaystyle {\ce {^3O2 ->[{\ce {light}}][{\ce {photosensitizer}}] ^1O2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/0a62c29558574cf534f0eaf188595d3f3c8bb29b)