|
| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Prop-2-enamide
| |||
| Other names
Acrylamide
Acrylic amide | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.001.067 | ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| Properties | |||
| C3H5NO | |||
| Molar mass | 71.079 g·mol−1 | ||
| Appearance | white crystalline solid, no odor | ||
| Density | 1.322 g/cm3 | ||
| Melting point | 84.5 °C (184.1 °F; 357.6 K) | ||
| Boiling point | None (polymerization); decomposes at 175-300°C | ||
| 2.04 kg/L (25 °C) | |||
| Hazards | |||
| Main hazards | potential occupational carcinogen | ||
| Safety data sheet | ICSC 0091 | ||
| GHS pictograms |  
| ||
| H301, H312, H315, H317, H319, H332, H340, H350, H361, H372 | |||
| P201, P280, P301+310, P305+351+338, P308+313 | |||
| NFPA 704 | |||
| Flash point | 138 °C (280 °F; 411 K) | ||
| 424 °C (795 °F; 697 K) | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
100-200 mg/kg (mammal, oral) 107 mg/kg (mouse, oral) 150 mg/kg (rabbit, oral) 150 mg/kg (guinea pig, oral) 124 mg/kg (rat, oral) | ||
| US health exposure limits (NIOSH): | |||
PEL (Permissible)
|
TWA 0.3 mg/m3 [skin] | ||
REL (Recommended)
|
Ca TWA 0.03 mg/m3 [skin] | ||
IDLH (Immediate danger)
|
60 mg/m3 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
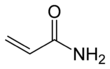
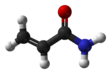
Acrylamide (or acrylic amide) is an organic compound with the chemical formula CH2=CHC(O)NH2. It is a white odorless solid, soluble in water and several organic solvents. It is produced industrially as a precursor to polyacrylamides, which find many uses as water-soluble thickeners and flocculation agents. It is highly toxic, likely to be carcinogenic, and partly for that reason it is mainly handled as an aqueous solution.
The discovery that some cooked foods contain acrylamide in 2002 attracted significant attention to its possible biological effects. As of 2019 epidemiological studies suggest it is unlikely that acrylamide consumption increases people's risk of developing cancer.
Production
Acrylamide can be prepared by the hydrolysis of acrylonitrile. The reaction is catalyzed by sulfuric acid as well as various metal salts. It is also catalyzed by the enzyme nitrile hydratase. US demand for acrylamide was 253,000,000 pounds (115,000,000 kg) as of
2007, increased from 245,000,000 pounds (111,000,000 kg) in 2006.
N-(D-glucos-1-yl)-L-asparagine, precursor to acrylamide in cooked food.
Acrylamide arises in some cooked foods via a series of steps initiated by the condensation of the amino acid asparagine and glucose. This condensation, one of the Maillard reactions followed by dehydrogenation produces N-(D-glucos-1-yl)-L-asparagine, which upon pyrolysis generates some acrylamide.
Uses
The majority of acrylamide is used to manufacture various polymers, especially polyacrylamide used as a thickening agent and in water treatment.
Toxicity and carcinogenicity
U.S. regulation
Acrylamide is classified as an extremely hazardous substance in the United States as defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act
(42 U.S.C. 11002), and is subject to strict reporting requirements by
facilities which produce, store, or use it in significant quantities.
Acrylamide is considered a potential occupational carcinogen by U.S. government agencies and classified as a Group 2A carcinogen by the IARC. The Occupational Safety and Health Administration and the National Institute for Occupational Safety and Health have set dermal occupational exposure limits at 0.03 mg/m3 over an eight-hour workday. In animal models, exposure to acrylamide causes tumors in the adrenal glands, thyroid, lungs, and testes.
Acrylamide is easily absorbed by the skin and distributed throughout
the organism; the highest levels of acrylamide post-exposure are found
in the blood, non-exposed skin, kidneys, liver, testes, and spleen.
Acrylamide can be metabolically-activated by cytochrome P450 to a
genotoxic metabolite, glycidamide,
which is considered to be a critical mode of action to the
carcinogenesis of acrylamide. On the other hand, acrylamide and
glycidamide can be detoxified via conjugation with glutathione to form
acrylamide- and isomeric glycidamide-glutathione conjugates, subsequently metabolized to mercapturic acids and excreted in urine. Acrylamide has also been found to have neurotoxic effects in humans who have been exposed. Animal studies show neurotoxic effects as well as mutations in sperm.
Hazards
Acrylamide is also a skin irritant and may be a tumor initiator in the skin, potentially increasing risk for skin cancer. Symptoms of acrylamide exposure include dermatitis in the exposed area, and peripheral neuropathy.
Laboratory research has found that some phytochemicals may have the potential to be developed into drugs which could alleviate the toxicity of acrylamide.
Occurrence in food and associated health risks
Discovery of acrylamide in foods
Hot french fries (chips) are heated to a high temperature
Acrylamide was discovered in foods in April 2002 by Eritrean scientist Eden Tareke in Sweden; she found the chemical in starchy foods such as potato chips (potato crisps), French fries
(chips), and bread that had been heated higher than 120 °C (248 °F).
Production of acrylamide in the heating process was shown to be
temperature dependent. It was not found in food that had been boiled, or in foods that were not heated.
Acrylamide has been found in roasted barley tea, called mugicha in Japanese. The barley
is roasted so it is dark brown prior to being steeped in hot water. The
roasting process produced 200–600 micrograms/kg of acrylamide in
mugicha.
This is less than the >1000 micrograms/kg found in potato crisps and
other fried whole potato snack foods cited in the same study and it is
unclear how much of this is ingested after the drink is prepared. Rice cracker and sweet potato
levels were lower than in potatoes. Potatoes cooked whole were found to
have significantly lower acrylamide levels than the others, suggesting a
link between food preparation method and acrylamide levels.
Acrylamide levels appear to rise as food is heated for longer
periods of time. Although researchers are still unsure of the precise
mechanisms by which acrylamide forms in foods, many believe it is a byproduct of the Maillard reaction. In fried or baked goods, acrylamide may be produced by the reaction between asparagine and reducing sugars (fructose, glucose, etc.) or reactive carbonyls at temperatures above 120 °C (248 °F).
The US FDA has analyzed a variety of U.S. food products for levels of acrylamide since 2002.
According to the EFSA, the main toxicity risks of acrylamide are "Neurotoxicity, adverse effects on male reproduction, developmental toxicity and carcinogenicity". However, according to their research, there is no concern on non-neoplastic
effects. Furthermore, while the relation between consumption of
acrylamide and cancer in rats and mice has been shown, it is still not
clear whether acrylamide consumption has an effect on the risk of
developing cancer in humans, and existing epidemological studies in humans are very limited and don't show any relation between acrylamide and cancer in humans. Food industry workers exposed to twice the average level of acrylamide do not exhibit higher cancer rates.
Acceptable limits
Although acrylamide has known toxic effects on the nervous system and on fertility, a June 2002 report by the Food and Agriculture Organization of the United Nations and the World Health Organization attempting to establish basic toxicology (threshold limit value, no-observed-adverse-effect levels, tolerable daily intake, etc.) concluded the intake level required to observe neuropathy
(0.5 mg/kg body weight/day) was 500 times higher than the average
dietary intake of acrylamide (1 μg/kg body weight/day). For effects on
fertility, the level is 2,000 times higher than the average intake.
From this, they concluded acrylamide levels in food were safe in terms
of neuropathy, but raised concerns over human carcinogenicity based on
known carcinogenicity in laboratory animals.
Opinions of health organizations
The American Cancer Society say that laboratory studies have shown that acrylamide is likely to be a carcinogen, but that as of 2019 evidence from epidemiological studies suggest that dietary acrylamide is unlikely to raise the risk of people developing cancer.
The World Health Organization
(WHO) has set up a clearinghouse for information about acrylamide that
includes a database of researchers and data providers; references for
research published elsewhere; information updates about the current
status of research efforts; and updates on information relevant to the
health risk of acrylamide in food.
HEATOX (heat-generated food toxicants) study in Europe
The Heat-generated Food Toxicants (HEATOX) Project was a European Commission-funded
multidisciplinary research project running from late 2003 to early
2007. Its objectives were to "estimate health risks that may be
associated with hazardous compounds in heat-treated food [, and to] find
cooking/processing methods that minimize the amounts of these
compounds, thereby providing safe, nutritious, and high-quality
food-stuffs." It found that "the evidence of acrylamide posing a cancer risk for humans has been strengthened,"
and that "compared with many regulated food carcinogens, the exposure
to acrylamide poses a higher estimated risk to European consumers."
HEATOX sought also to provide consumers with advice on how to lower
their intake of acrylamide, specifically pointing out that home-cooked
food tends to contribute far less to overall acrylamide levels than food
that was industrially prepared, and that avoiding overcooking is one of
the best ways to minimize exposure at home.
Public awareness
On April 24, 2002, the Swedish National Food Administration announced that acrylamide can be found in baked and fried starchy foods, such as potato chips, breads, and cookies. Concern was raised mainly because of the probable carcinogenic effects of acrylamide. This was followed by a strong, but short-lived, interest from the press.
On August 26, 2005, California attorney general Bill Lockyer filed a lawsuit against four makers of french fries and potato chips – H.J. Heinz Co., Frito-Lay, Kettle Foods Inc., and Lance Inc. – to reduce the risk to consumers from consuming acrylamide.
The lawsuit was settled on August 1, 2008, with the food producers
agreeing to cut acrylamide levels to 275 parts per billion in three
years, to avoid a Proposition 65 warning label.
The companies avoided trial by agreeing to pay a combined $3 million in
fines as a settlement with the California attorney general's office.
In 2016, the UK Food Standards Agency
launched a campaign called "Go for Gold", warning of the possible
cancer risk associated with cooking potatoes and other starchy foods at
high temperatures.
In 2018, a judge in California ruled that the coffee
industry had not provided sufficient evidence that acrylamide contents
in coffee were at safe enough levels to not require a Proposition 65
warning.
Occurrence in cigarettes
Cigarette smoking is a major acrylamide source. It has been shown in one study to cause an increase in blood acrylamide levels three-fold greater than any dietary factor.