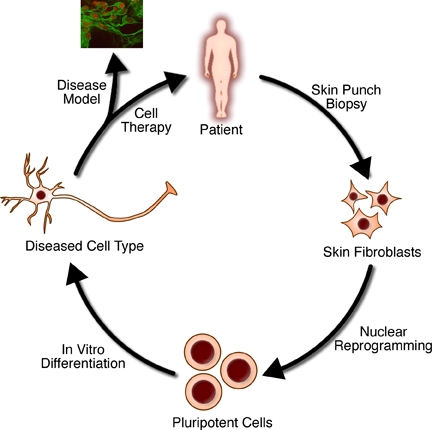
A simplified overview of the general methods used in regenerative medicine
Tissue engineering is the use of a combination of cells, engineering, and materials methods, and suitable biochemical and physicochemical factors to improve or replace biological tissues. Tissue engineering involves the use of a tissue scaffold for the formation of new viable tissue for a medical purpose. While it was once categorized as a sub-field of biomaterials, having grown in scope and importance it can be considered as a field in its own.
While most definitions of tissue engineering cover a broad range of
applications, in practice the term is closely associated with
applications that repair or replace portions of or whole tissues (i.e., bone, cartilage,[1] blood vessels, bladder, skin, muscle
etc.). Often, the tissues involved require certain mechanical and
structural properties for proper functioning. The term has also been
applied to efforts to perform specific biochemical functions using cells within an artificially-created support system (e.g. an artificial pancreas, or a bio artificial liver). The term regenerative medicine is often used synonymously with tissue engineering, although those involved in regenerative medicine place more emphasis on the use of stem cells or progenitor cells to produce tissues.
Overview
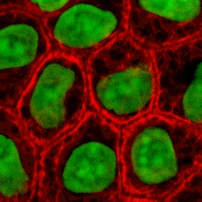
Micro-mass cultures of C3H-10T1/2 cells at varied oxygen tensions stained with Alcian blue
A commonly applied definition of tissue engineering, as stated by Langer and Vacanti, is "an interdisciplinary
field that applies the principles of engineering and life sciences
toward the development of biological substitutes that restore, maintain,
or improve [Biological tissue] function or a whole organ".
Tissue engineering has also been defined as "understanding the
principles of tissue growth, and applying this to produce functional
replacement tissue for clinical use".
A further description goes on to say that an "underlying supposition of
tissue engineering is that the employment of natural biology of the
system will allow for greater success in developing therapeutic
strategies aimed at the replacement, repair, maintenance, or enhancement
of tissue function".
Powerful developments in the multidisciplinary field of tissue
engineering have yielded a novel set of tissue replacement parts and
implementation strategies. Scientific advances in biomaterials, stem cells, growth and differentiation factors, and biomimetic
environments have created unique opportunities to fabricate tissues in
the laboratory from combinations of engineered extracellular matrices
("scaffolds"), cells, and biologically active molecules. Among the major
challenges now facing tissue engineering is the need for more complex
functionality, as well as both functional and biomechanical stability
and vascularization in laboratory-grown tissues destined for
transplantation. The continued success of tissue engineering and the
eventual development of true human replacement parts will grow from the
convergence of engineering and basic research advances in tissue,
matrix, growth factor, stem cell, and developmental biology, as well as
materials science and bioinformatics...
In 2003, the NSF
published a report entitled "The Emergence of Tissue Engineering as a
Research Field", which gives a thorough description of the history of
this field.
Examples
Regenerating a human ear using a scaffold
- Bioartificial windpipe: The first procedure of regenerative medicine of an implantation of a "bioartificial" organ.
- In vitro meat: Edible artificial animal muscle tissue cultured in vitro.
- Bioartificial liver device: several research efforts have produced hepatic assist devices utilizing living hepatocytes.
- Artificial pancreas: research involves using islet cells to produce and regulate insulin, particularly in cases of diabetes.
- Artificial bladders: Anthony Atala (Wake Forest University) has successfully implanted artificially grown bladders into seven out of approximately 20 human test subjects as part of a long-term experiment.
- Cartilage: lab-grown tissue was successfully used to repair knee cartilage.
- Scaffold-free cartilage: Cartilage generated without the use of exogenous scaffold material. In this methodology, all material in the construct is cellular or material produced directly by the cells themselves.
- Doris Taylor's heart in a jar
- Tissue-engineered airway
- Tissue-engineered vessels
- Artificial skin constructed from human skin cells embedded in a hydrogel, such as in the case of bioprinted constructs for battlefield burn repairs.
- Artificial bone marrow
- Artificial bone
- Laboratory-grown penis
- Oral mucosa tissue engineering
- Foreskin
Cells as building blocks
Stained cells in culture
Tissue engineering utilizes living cells as engineering materials. Examples include using living fibroblasts in skin replacement or repair, cartilage repaired with living chondrocytes, or other types of cells used in other ways.
Cells became available as engineering materials when scientists at Geron Corp. discovered how to extend telomeres in 1998, producing immortalized cell lines. Before this, laboratory cultures of healthy, noncancerous mammalian cells would only divide a fixed number of times, up to the Hayflick limit, before dying.
Extraction
From fluid tissues such as blood, cells are extracted by bulk methods, usually centrifugation or apheresis. From solid tissues, extraction is more difficult. Usually, the tissue is minced and then digested with the enzymes trypsin or collagenase to remove the extracellular matrix (ECM) that holds the cells. After that, the cells are free floating, and extracted using centrifugation or apheresis.
Digestion with trypsin is very dependent on temperature. Higher temperatures digest the matrix faster but create more damage. Collagenase is less temperature dependent, and damages fewer cells, but takes longer and is a more expensive reagent.
Types of cells
Cells are often categorized by their source
Autologous
cells are obtained from the same individual to which they will be
reimplanted. Autologous cells have the fewest problems with rejection
and pathogen transmission, however, in some cases might not be
available. For example, in genetic disease
suitable autologous cells are not available. Also, very ill or elderly
persons, as well as patients suffering from severe burns, may not have
sufficient quantities of autologous cells to establish useful cell
lines. Moreover, since this category of cells needs to be harvested from
the patient, there are also some concerns related to the necessity of
performing such surgical operations that might lead to donor site
infection or chronic pain. Autologous cells also must be cultured from
samples before they can be used: this takes time, so autologous
solutions may not be very quick. Recently there has been a trend towards
the use of mesenchymal stem cells from bone marrow and fat. These cells can differentiate into a variety of tissue types, including bone, cartilage, fat, and nerve.
A large number of cells can be easily and quickly isolated from fat,
thus opening the potential for large numbers of cells to be quickly and
easily obtained.
Allogeneic cells come from the body of a donor of the same
species. While there are some ethical constraints to the use of human
cells for in vitro studies, the employment of dermal fibroblasts from human foreskin has been demonstrated to be immunologically safe and thus a viable choice for tissue engineering of skin.
Xenogenic cells are these isolated from individuals of
another species. In particular animal cells have been used quite
extensively in experiments aimed at the construction of cardiovascular
implants.
Syngenic or isogenic cells are isolated from genetically identical organisms, such as twins, clones, or highly inbred research animal models.
Primary cells are from an organism.
Secondary cells are from a cell bank.
Stem cells
are undifferentiated cells with the ability to divide in culture and
give rise to different forms of specialized cells. According to their
source stem cells are divided into "adult" and "embryonic" stem cells,
the first class being multipotent and the latter mostly pluripotent; some cells are totipotent,
in the earliest stages of the embryo. While there is still a large
ethical debate related with the use of embryonic stem cells, it is
thought that another alternative source - induced stem cells may be useful for the repair of diseased or damaged tissues, or may be used to grow new organs.
Scaffolds
Scaffolds
are materials that have been engineered to cause desirable cellular
interactions to contribute to the formation of new functional tissues
for medical purposes. Cells are often 'seeded' into these structures
capable of supporting three-dimensional tissue formation. Scaffolds mimic the extracellular matrix of the native tissue, recapitulating the in vivo
milieu and allowing cells to influence their own microenvironments.
They usually serve at least one of the following purposes: allow cell
attachment and migration, deliver and retain cells and biochemical
factors, enable diffusion of vital cell nutrients and expressed
products, exert certain mechanical and biological influences to modify
the behaviour of the cell phase.
In 2009, an interdisciplinary team led by the thoracic surgeon Thorsten Walles
implanted the first bioartificial transplant that provides an innate
vascular network for post-transplant graft supply successfully into a
patient awaiting tracheal reconstruction.
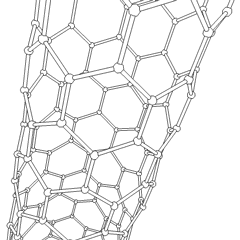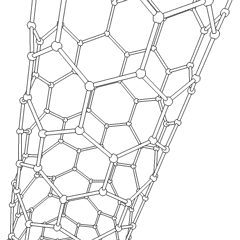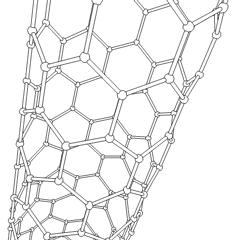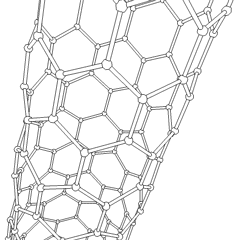
This animation of a rotating carbon nanotube shows its 3D structure. Carbon nanotubes are among the numerous candidates for tissue engineering scaffolds since they are biocompatible, resistant to biodegradation and can be functionalized with biomolecules. However, the possibility of toxicity with non-biodegradable nano-materials is not fully understood.
To achieve the goal of tissue reconstruction, scaffolds must meet
some specific requirements. High porosity and adequate pore size are
necessary to facilitate cell seeding and diffusion throughout the whole
structure of both cells and nutrients. Biodegradability
is often an essential factor since scaffolds should preferably be
absorbed by the surrounding tissues without the necessity of surgical
removal. The rate at which degradation occurs has to coincide as much as
possible with the rate of tissue formation: this means that while cells
are fabricating their own natural matrix structure around themselves,
the scaffold is able to provide structural integrity within the body and
eventually it will break down leaving the newly formed tissue which
will take over the mechanical load. Injectability is also important for
clinical uses.
Recent research on organ printing is showing how crucial a good control
of the 3D environment is to ensure reproducibility of experiments and
offer better results.
Materials
Many different materials (natural and synthetic, biodegradable and permanent) have been investigated.
Most of these materials have been known in the medical field before the
advent of tissue engineering as a research topic, being already
employed as bioresorbable sutures. Examples of these materials are collagen and some polyesters.
New biomaterials have been engineered to have ideal properties
and functional customization: injectability, synthetic manufacture, biocompatibility,
non-immunogenicity, transparency, nano-scale fibers, low concentration,
resorption rates, etc. PuraMatrix, originating from the MIT labs of
Zhang, Rich, Grodzinsky, and Langer is one of these new biomimetic
scaffold families which has now been commercialized and is impacting
clinical tissue engineering.
A commonly used synthetic material is PLA - polylactic acid. This is a polyester which degrades within the human body to form lactic acid, a naturally occurring chemical which is easily removed from the body. Similar materials are polyglycolic acid (PGA) and polycaprolactone
(PCL): their degradation mechanism is similar to that of PLA, but they
exhibit respectively a faster and a slower rate of degradation compared
to PLA. While these materials have well maintained mechanical strength
and structural integrity, they exhibit a hydrophobic nature. This
hydrophobicity inhibits their biocompatibility, which makes them less
effective for in vivo use as tissue scaffolding.
In order to fix the lack of biocompatibility, much research has been
done to combine these hydrophobic materials with hydrophilic and more
biocompatible hydrogels. While these hydrogels have a superior
biocompatibility, they lack the structural integrity of PLA, PCL, and
PGA. By combining the two different types of materials, researchers are
trying to create a synergistic relationship that produces a more
biocompatible tissue scaffolding.
Scaffolds may also be constructed from natural materials: in particular different derivatives of the extracellular matrix have been studied to evaluate their ability to support cell growth. Proteic materials, such as collagen or fibrin, and polysaccharidic materials, like chitosan or glycosaminoglycans
(GAGs), have all proved suitable in terms of cell compatibility, but
some issues with potential immunogenicity still remains. Among GAGs hyaluronic acid, possibly in combination with cross linking agents (e.g. glutaraldehyde, water-soluble carbodiimide,
etc.), is one of the possible choices as scaffold material.
Functionalized groups of scaffolds may be useful in the delivery of
small molecules (drugs) to specific tissues. Another form of scaffold
under investigation is decellularised tissue extracts whereby the
remaining cellular remnants/extracellular matrices act as the scaffold.
Recently a range of nanocomposites biomaterials are fabricated by incorporating nanomaterials within the polymeric matrix to engineer bioactive scaffolds.
A 2009 study by Derda et al. aimed to improve in vivo-like
conditions for 3D tissue via "stacking and de-stacking layers of paper
impregnated with suspensions of cells in extracellular matrix hydrogel, making it possible to control oxygen and nutrient gradients in 3D, and to analyze molecular and genetic responses". It is possible to manipulate gradients of soluble
molecules, and to characterize cells in these complex gradients more
effectively than conventional 3D cultures based on hydrogels, cell
spheroids, or 3D perfusion reactors.
Different thicknesses of paper and types of medium can support a
variety of experimental environments. Upon deconstruction, these sheets
can be useful in cell-based high-throughput screening and drug discovery.
Synthesis
Tissue engineered vascular graft
Tissue engineered heart valve
A number of different methods have been described in the literature
for preparing porous structures to be employed as tissue engineering
scaffolds. Each of these techniques presents its own advantages, but
none are free of drawbacks.
Nanofiber self-assembly
Molecular
self-assembly is one of the few methods for creating biomaterials with
properties similar in scale and chemistry to that of the natural in vivo extracellular matrix (ECM), a crucial step toward tissue engineering of complex tissues.
Moreover, these hydrogel scaffolds have shown superiority in in vivo
toxicology and biocompatibility compared to traditional macroscaffolds
and animal-derived materials.
Textile technologies
These techniques include all the approaches that have been successfully employed for the preparation of non-woven meshes of different polymers. In particular, non-woven polyglycolide
structures have been tested for tissue engineering applications: such
fibrous structures have been found useful to grow different types of
cells. The principal drawbacks are related to the difficulties in
obtaining high porosity and regular pore size.
Solvent casting and particulate leaching
Solvent casting and particulate leaching
(SCPL) allows for the preparation of structures with regular porosity,
but with limited thickness. First, the polymer is dissolved into a
suitable organic solvent (e.g. polylactic acid could be dissolved into dichloromethane), then the solution is cast into a mold filled with porogen particles. Such porogen can be an inorganic salt like sodium chloride, crystals of saccharose, gelatin spheres or paraffin
spheres. The size of the porogen particles will affect the size of the
scaffold pores, while the polymer to porogen ratio is directly
correlated to the amount of porosity of the final structure. After the
polymer solution has been cast the solvent is allowed to fully
evaporate, then the composite structure in the mold is immersed in a
bath of a liquid suitable for dissolving the porogen: water in the case
of sodium chloride, saccharose and gelatin or an aliphatic solvent like hexane
for use with paraffin. Once the porogen has been fully dissolved, a
porous structure is obtained. Other than the small thickness range that
can be obtained, another drawback of SCPL lies in its use of organic
solvents which must be fully removed to avoid any possible damage to the
cells seeded on the scaffold.
Gas foaming
To
overcome the need to use organic solvents and solid porogens, a
technique using gas as a porogen has been developed. First, disc-shaped
structures made of the desired polymer are prepared by means of
compression molding using a heated mold. The discs are then placed in a
chamber where they are exposed to high pressure CO2
for several days. The pressure inside the chamber is gradually restored
to atmospheric levels. During this procedure the pores are formed by
the carbon dioxide molecules that abandon the polymer, resulting in a
sponge-like structure. The main problems resulting from such a technique
are caused by the excessive heat used during compression molding (which
prohibits the incorporation of any temperature labile material into the
polymer matrix) and by the fact that the pores do not form an
interconnected structure.
Emulsification freeze-drying
This
technique does not require the use of a solid porogen like SCPL. First,
a synthetic polymer is dissolved into a suitable solvent (e.g.
polylactic acid in dichloromethane) then water is added to the polymeric
solution and the two liquids are mixed in order to obtain an emulsion. Before the two phases can separate, the emulsion is cast into a mold and quickly frozen by means of immersion into liquid nitrogen. The frozen emulsion is subsequently freeze-dried
to remove the dispersed water and the solvent, thus leaving a
solidified, porous polymeric structure. While emulsification and
freeze-drying allow for a faster preparation when compared to SCPL
(since it does not require a time-consuming leaching step), it still
requires the use of solvents. Moreover, pore size is relatively small
and porosity is often irregular. Freeze-drying by itself is also a
commonly employed technique for the fabrication of scaffolds. In
particular, it is used to prepare collagen sponges: collagen is
dissolved into acidic solutions of acetic acid or hydrochloric acid that are cast into a mold, frozen with liquid nitrogen and then lyophilized.
Thermally induced phase separation
Similar
to the previous technique, the TIPS phase separation procedure requires
the use of a solvent with a low melting point that is easy to sublime.
For example, dioxane
could be used to dissolve polylactic acid, then phase separation is
induced through the addition of a small quantity of water: a
polymer-rich and a polymer-poor phase are formed. Following cooling
below the solvent melting point and some days of vacuum-drying to
sublime the solvent, a porous scaffold is obtained. Liquid-liquid phase
separation presents the same drawbacks of emulsification/freeze-drying.
Electrospinning
Electrospinning
is a highly versatile technique that can be used to produce continuous
fibers from submicrometer to nanometer diameters. In a typical
electrospinning set-up, a solution is fed through a spinneret and a high
voltage is applied to the tip. The buildup of electrostatic repulsion
within the charged solution, causes it to eject a thin fibrous stream. A
mounted collector plate or rod with an opposite or grounded charge
draws in the continuous fibers, which arrive to form a highly porous
network. The primary advantages of this technique are its simplicity and
ease of variation. At a laboratory level, a typical electrospinning
set-up only requires a high voltage power supply (up to 30 kV), a
syringe, a flat tip needle, and a conducting collector. For these
reasons, electrospinning has become a common method of scaffold
manufacture in many labs. By modifying variables such as the distance to
collector, magnitude of applied voltage, or solution flow
rate—researchers can dramatically change the overall scaffold
architecture.
Historically, research on electrospun fibrous scaffolds dates
back to at least the late 1980s when Simon showed that electrospinning
could be used to produced nano- and submicron-scale fibrous scaffolds
from polymer solutions specifically intended for use as in vitro
cell and tissue substrates. This early use of electrospun lattices for
cell culture and tissue engineering showed that various cell types would
adhere to and proliferate upon polycarbonate fibers. It was noted that
as opposed to the flattened morphology typically seen in 2D culture,
cells grown on the electrospun fibers exhibited a more rounded
3-dimensional morphology generally observed of tissues in vivo.
CAD/CAM technologies
Because most of the above techniques are limited when it comes to the control of porosity and pore size, computer assisted design and manufacturing
techniques have been introduced to tissue engineering. First, a
three-dimensional structure is designed using CAD software. The porosity
can be tailored using algorithms within the software. The scaffold is then realized by using ink-jet printing of polymer powders or through Fused Deposition Modeling of a polymer melt.
A 2011 study by El-Ayoubi et al. investigated "3D-plotting technique to produce (biocompatible and biodegradable)
poly-L-Lactide macroporous scaffolds with two different pore sizes" via
solid free-form fabrication (SSF) with computer-aided-design (CAD), to
explore therapeutic articular cartilage replacement as an "alternative to conventional tissue repair".
The study found the smaller the pore size paired with mechanical stress
in a bioreactor (to induce in vivo-like conditions), the higher the
cell viability in potential therapeutic functionality via decreasing
recovery time and increasing transplant effectiveness.
Laser-assisted bioprinting
In a 2012 study,
Koch et al. focused on whether Laser-assisted BioPrinting (LaBP) can be
used to build multicellular 3D patterns in natural matrix, and whether
the generated constructs are functioning and forming tissue. LaBP
arranges small volumes of living cell suspensions in set high-resolution
patterns. The investigation was successful, the researchers foresee that "generated tissue constructs might be used for in vivo testing by implanting them into animal models". As of this study, only human skin tissue has been synthesized,
though researchers project that "by integrating further cell types (e.g.
melanocytes, Schwann cells, hair follicle cells) into the printed cell construct, the behavior of these cells in a 3D in vitro microenvironment similar to their natural one can be analyzed", which is useful for drug discovery and toxicology studies.
Assembly methods
One
of the continuing, persistent problems with tissue engineering is mass
transport limitations. Engineered tissues generally lack an initial
blood supply, thus making it difficult for any implanted cells to obtain
sufficient oxygen and nutrients to survive, or function properly.
Self-assembly
Self-assembly
methods have been shown to be promising methods for tissue engineering.
Self-assembly methods have the advantage of allowing tissues to develop
their own extracellular matrix, resulting in tissue that better
recapitulates biochemical and biomechanical properties of native tissue.
Self-assembling engineered articular cartilage was introduced by Jerry
Hu and Kyriacos A. Athanasiou in 2006 and applications of the process have resulted in engineered cartilage approaching the strength of native tissue.
Self-assembly is a prime technology to get cells grown in a lab to
assemble into three-dimensional shapes. To break down tissues into
cells, researchers first have to dissolve the extracellular matrix that
normally binds them together. Once cells are isolated, they must form
the complex structures that make up our natural tissues.
Liquid-based template assembly
The air-liquid surface established by Faraday waves
is explored as a template to assemble biological entities for bottom-up
tissue engineering. This liquid-based template can be dynamically
reconfigured in a few seconds, and the assembly on the template can be
achieved in a scalable and parallel manner. Assembly of microscale
hydrogels, cells, neuron-seeded micro-carrier beads, cell spheroids into
various symmetrical and periodic structures was demonstrated with good
cell viability. Formation of 3D neural network was achieved after 14-day
tissue culture.
Additive manufacturing
It might be possible to print organs, or possibly entire organisms using additive manufacturing
techniques. A recent innovative method of construction uses an ink-jet
mechanism to print precise layers of cells in a matrix of
thermoreversible gel. Endothelial cells, the cells that line blood
vessels, have been printed in a set of stacked rings. When incubated,
these fused into a tube.
The field of three-dimensional and highly accurate models of
biological systems is pioneered by multiple projects and technologies
including a rapid method for creating tissues and even whole organs
involve a 3D printer that can print the scaffolding and cells layer by
layer into a working tissue sample or organ. The device is presented in a
TED talk by Dr. Anthony Atala, M.D. the Director of the Wake Forest Institute for Regenerative Medicine, and the W.H. Boyce Professor and Chair of the Department of Urology at Wake Forest University, in which a kidney is printed on stage during the seminar and then presented to the crowd.
It is anticipated that this technology will enable the production of
livers in the future for transplantation and theoretically for toxicology and other biological studies as well.
Recently Multi-Photon Processing (MPP) was employed for in vivo
experiments by engineering artificial cartilage constructs. An ex vivo
histological examination showed that certain pore geometry and the
pre-growing of chondrocytes (Cho) prior to implantation significantly
improves the performance of the created 3D scaffolds. The achieved
biocompatibility was comparable to the commercially available collagen
membranes. The successful outcome of this study supports the idea that
hexagonal-pore-shaped hybrid organic-inorganic microstructured scaffolds
in combination with Cho seeding may be successfully implemented for
cartilage tissue engineering.
Scaffolding
In 2013, using a 3-d scaffolding of Matrigel in various configurations, substantial pancreatic organoids
was produced in vitro. Clusters of small numbers of cells proliferated
into 40,000 cells within one week. The clusters transform into cells
that make either digestive enzymes or hormones like insulin, self-organizing into branched pancreatic organoids that resemble the pancreas.
The cells are sensitive to the environment, such as gel stiffness
and contact with other cells. Individual cells do not thrive; a minimum
of four proximate cells was required for subsequent organoid
development. Modifications to the medium composition produced either
hollow spheres mainly composed of pancreatic progenitors, or complex
organoids that spontaneously undergo pancreatic morphogenesis and
differentiation. Maintenance and expansion of pancreatic progenitors
require active Notch and FGF signaling, recapitulating in vivo niche signaling interactions.
The organoids were seen as potentially offering mini-organs for drug testing and for spare insulin-producing cells.
Tissue culture
In many cases, creation of functional tissues and biological structures in vitro requires extensive culturing
to promote survival, growth and inducement of functionality. In
general, the basic requirements of cells must be maintained in culture,
which include oxygen, pH, humidity, temperature, nutrients and osmotic pressure maintenance.
Tissue engineered cultures also present additional problems in maintaining culture conditions. In standard cell culture, diffusion
is often the sole means of nutrient and metabolite transport. However,
as a culture becomes larger and more complex, such as the case with
engineered organs and whole tissues, other mechanisms must be employed
to maintain the culture, such as the creation of capillary networks
within the tissue.
Bioreactor for cultivation of vascular grafts
Another issue with tissue culture is introducing the proper factors
or stimuli required to induce functionality. In many cases, simple
maintenance culture is not sufficient. Growth factors, hormones,
specific metabolites or nutrients, chemical and physical stimuli are
sometimes required. For example, certain cells respond to changes in
oxygen tension as part of their normal development, such as chondrocytes, which must adapt to low oxygen conditions or hypoxia during skeletal development. Others, such as endothelial cells, respond to shear stress from fluid flow, which is encountered in blood vessels.
Mechanical stimuli, such as pressure pulses seem to be beneficial to
all kind of cardiovascular tissue such as heart valves, blood vessels or
pericardium.
Bioreactors
A bioreactor in tissue engineering, as opposed to industrial
bioreactors, is a device that attempts to simulate a physiological
environment in order to promote cell or tissue growth in vitro. A
physiological environment can consist of many different parameters such
as temperature and oxygen or carbon dioxide concentration but can extend
to all kinds of biological, chemical or mechanical stimuli. Therefore,
there are systems that may include the application of forces or stresses
to the tissue or even of electric current in two- or three-dimensional
setups.
In academic and industry research facilities, it is typical for
bioreactors to be developed to replicate the specific physiological
environment of the tissue being grown (e.g., flex and fluid shearing for
heart tissue growth).
Several general-use and application-specific bioreactors are also
commercially available, and may provide static chemical stimulation or
combination of chemical and mechanical stimulation.
There are a variety of Bioreactors
designed for 3D cell cultures. There are small plastic cylindrical
chambers, as well as glass chambers, with regulated internal humidity
and moisture specifically engineered for the purpose of growing cells in
three dimensions. The bioreactor uses bioactive synthetic materials such as polyethylene terephthalate membranes to surround the spheroid cells in an environment that maintains high levels of nutrients.
They are easy to open and close, so that cell spheroids can be removed
for testing, yet the chamber is able to maintain 100% humidity
throughout.
This humidity is important to achieve maximum cell growth and function.
The bioreactor chamber is part of a larger device that rotates to
ensure equal cell growth in each direction across three dimensions.
QuinXell Technologies from Singapore
has developed a bioreactor known as the TisXell Biaxial Bioreactor
which is specially designed for the purpose of tissue engineering. It is
the first bioreactor in the world to have a spherical glass chamber
with biaxial
rotation; specifically to mimic the rotation of the fetus in the womb;
which provides a conducive environment for the growth of tissues.
MC2 Biotek has also developed a bioreactor known as ProtoTissue that uses gas exchange
to maintain high oxygen levels within the cell chamber; improving upon
previous bioreactors, because the higher oxygen levels help the cell
grow and undergo normal cell respiration.
Long fiber generation
In 2013, a group from the University of Tokyo developed cell laden fibers up to a meter in length and on the order of 100 µm in size. These fibers were created using a microfluidic device that forms a double coaxial laminar flow. Each 'layer' of the microfluidic device (cells seeded in ECM,
a hydrogel sheath, and finally a calcium chloride solution). The seeded
cells culture within the hydrogel sheath for several days, and then the
sheath is removed with viable cell fibers. Various cell types were
inserted into the ECM core, including myocytes, endothelial cells, nerve cell fibers, and epithelial cell
fibers. This group then showed that these fibers can be woven together
to fabricate tissues or organs in a mechanism similar to textile weaving.
Fibrous morphologies are advantageous in that they provide an
alternative to traditional scaffold design, and many organs (such as
muscle) are composed of fibrous cells.
Bioartificial organs
An artificial organ is a man-made device that is implanted or
integrated into a human to replace a natural organ, for the purpose of
restoring a specific function or a group of related functions so the
patient may return to normal life as soon as possible. The replaced
function doesn't necessarily have to be related to life support but
often is. The ultimate goal of tissue engineering as a discipline is to
allow both 'off the shelf' bioartificial organs and regeneration of
injured tissue in the body. In order to successfully create
bioartificial organs from patients stem cells, researchers continue to
make improvements in the generation of complex tissues by tissue
engineering. For example, much research is aimed at understanding
nanoscale cues present in a cell’s microenvironment.
Biomimetics
Biomimetics is a field that aims to produce materials and systems that replicate those present in nature.
In the context of tissue engineering, this is a common approach used by
engineers to create materials for these applications that are
comparable to native tissues in terms of their structure, properties,
and biocompatibility. Material properties are largely dependent on
physical, structural, and chemical characteristics of that material.
Subsequently, a biomimetic approach to system design will become
significant in material integration, and a sufficient understanding of
biological processes and interactions will be necessary. Replication of
biological systems and processes may also be used in the synthesis of
bio-inspired materials to achieve conditions that produce the desired
biological material. Therefore, if a material is synthesized having the
same characteristics of biological tissues both structurally and
chemically, then ideally the synthesized material will have similar
properties. This technique has an extensive history originating from the
idea of using natural phenomenon as design inspiration for solutions to
human problems. Many modern advancements in technology have been
inspired by nature and natural systems, including aircraft, automobiles,
architecture, and even industrial systems. Advancements in
nanotechnology initiated the application of this technique to micro- and
nano-scale
problems, including tissue engineering. This technique has been used to
develop synthetic bone tissues, vascular technologies, scaffolding
materials and integration techniques, and functionalized nanoparticles.
Constructing neural networks in soft material
In
2018, scientists at Brandeis University reported their research on soft
material embedded with chemical networks which can mimic the smooth and
coordinated behavior of neural tissue. This research was funded by the U.S. Army Research Laboratory. The researchers presented an experimental system of neural networks, theoretically modeled as reaction-diffusion systems. Within the networks was an array of patterned reactors, each performing the Belousov-Zhabotinsky (BZ) reaction. These reactors could function on a nanoliter scale.
The researchers state that the inspiration for their project was the movement of the blue ribbon eel. The eel's movements are controlled by electrical impulses determined by a class of neural networks called the central pattern generator. Central Pattern Generators function within the autonomic nervous system to control bodily functions such as respiration, movement, and peristalsis.
Qualities of the reactor that were designed were the network topology, boundary conditions,
initial conditions, reactor volume, coupling strength, and the synaptic
polarity of the reactor (whether its behavior is inhibitory or
excitatory). A BZ emulsion system with a solid elastomer polydimethylsiloxane
(PDMS) was designed. Both light and bromine permeable PDMS have been
reported as viable methods to create a pacemaker for neural networks.