| Plasmodium falciparum | |
|---|---|

| |
| Macrogametocyte (left) and microgametocyte (right) of P. falciparum | |
| Scientific classification | |
| (unranked): | Diaphoretickes |
| Clade: | TSAR |
| Clade: | SAR |
| Infrakingdom: | Alveolata |
| Phylum: | Apicomplexa |
| Class: | Aconoidasida |
| Order: | Haemospororida |
| Family: | Plasmodiidae |
| Genus: | Plasmodium |
| Species: |
P. falciparum
|
| Binomial name | |
| Plasmodium falciparum
Welch, 1897
| |
| Synonyms | |
| |
Plasmodium falciparum is a unicellular protozoan parasite of humans, and the deadliest species of Plasmodium that causes malaria in humans. The parasite is transmitted through the bite of a female Anopheles mosquito and causes the disease's most dangerous form, falciparum malaria. It is responsible for around 50% of all malaria cases. P. falciparum is therefore regarded as the deadliest parasite in humans, causing 405,000 deaths in 2018. It is also associated with the development of blood cancer (Burkitt's lymphoma) and is classified as Group 2A carcinogen.
The species originated from the malarial parasite Laverania found in gorillas, around 10,000 years ago. Alphonse Laveran was the first to identify the parasite in 1880, and named it Oscillaria malariae. Ronald Ross discovered its transmission by mosquito in 1897. Giovanni Battista Grassi elucidated the complete transmission from a female anopheline mosquito to humans in 1898. In 1897, William H. Welch created the name Plasmodium falciparum, which ICZN formally adopted in 1954. P. falciparum assumes several different forms during its life cycle. The human-infective stage are sporozoites from the salivary gland of a mosquito. The sporozoites grow and multiply in the liver to become merozoites. These merozoites invade the erythrocytes (RBCs) to form trophozoites, schizonts and gametocytes, during which the symptoms of malaria are produced. In the mosquito, the gametocytes undergo sexual reproduction to a zygote, which turns into ookinete. Ookinete forms oocyts from which sporozoites are formed.
As of the World Health Organization World Malaria Report 2019, there were 228 million cases of malaria worldwide in 2018, resulting in an estimated 405,000 deaths. Nearly all malarial deaths are caused by P. falciparum, and 94% of such cases occur in Africa. Children under five years of age are most affected, accounting for 61% of the total deaths. In Sub-Saharan Africa, over 75% of cases were due to P. falciparum, whereas in most other malarial countries, other, less virulent plasmodial species predominate.
History
Laveran's drawing of various stages of P. falciparum as seen on fresh blood (1880).
Falciparum malaria was familiar to the ancient Greeks, who gave the general name πυρετός pyretós "fever". Hippocrates (c. 460–370 BCE) gave several descriptions on tertian fever and quartan fever. It was prevalent throughout the ancient Egyptian and Roman civilizations. It was the Romans who named the disease "malaria"—mala for bad, and aria for air, as they believed that the disease was spread by contaminated air, or miasma.
Discovery
A German physician, Johann Friedrich Meckel, must have been the first to see P. falciparum
but without knowing what it was. In 1847 he reported the presence of
black pigment granules from the blood and spleen of a patient who died
of malaria. The French Army physician Charles Louis Alphonse Laveran, while working at Bône Hospital (now Annaba
in Algeria), correctly identified the parasite as a causative pathogen
of malaria in 1880. He presented his discovery before the French Academy of Medicine in Paris, and published it in The Lancet, in 1881. He gave the scientific name Oscillaria malariae. But his discovery was received with skepticism mainly because by that time leading physicians such as Theodor Albrecht Edwin Klebs and Corrado Tommasi-Crudeli claimed that they had discovered a bacterium (which they called Bacillus malariae) as the pathogen of malaria. Laveran's discovery was widely accepted only after five years when Camillo Golgi
confirmed the parasite using better microscope and staining technique.
Laveran was awarded the Nobel Prize in Physiology or Medicine in 1907
for his work. In 1900, the Italian zoologist Giovanni Battista Grassi categorized Plasmodium species based on the timing of fever in the patient; malignant tertian malaria was caused by Laverania malariae (now P. falciparum), benign tertian malaria by Haemamoeba vivax (now P. vivax), and quartan malaria by Haemamoeba malariae (now P. malariae).
The British physician Patrick Manson formulated the mosquito-malaria theory in 1894; until that time, malarial parasites were believed to be spread in air as miasma, a Greek word for pollution. His colleague Ronald Ross,
a British Army surgeon, travelled to India to test the theory. Ross
discovered in 1897 that malarial parasites lived in certain mosquitoes.
The next year, he demonstrated that a malarial parasite of birds could
be transmitted by mosquitoes from one bird to another. Around the same
time, Grassi demonstrated that P. falciparum was transmitted in humans only by female anopheline mosquito (in his case Anopheles claviger).
Ross, Manson and Grassi were nominated for the Nobel Prize in
Physiology or Medicine in 1902. Under controversial circumstances, only
Ronald Ross was selected for the award.
There was a long debate on the taxonomy. It was only in 1954 the International Commission on Zoological Nomenclature officially approved the binominal Plasmodium falciparum. The valid genus Plasmodium was created by two Italian physicians Ettore Marchiafava and Angelo Celli in 1885. The species name was introduced by an American physician William Henry Welch in 1897. It is derived from the Latin falx, meaning "sickle" and parum meaning "like or equal to another".
Origin and evolution
P. falciparum is now generally accepted to have evolved from Laverania (a subgenus of Plasmodium found in apes) species present in gorilla in Western Africa. Genetic diversity indicates that the human protozoan emerged around 10,000 years ago. The closest relative of P. falciparum is P. praefalciparum, a parasite of gorillas, as supported by mitochondrial, apicoplastic and nuclear DNA sequences. These two species are closely related to the chimpanzee parasite P. reichenowi, which was previously thought to be the closest relative of P. falciparum. P. falciparum was also once thought to originate from a parasite of birds.
Levels of genetic polymorphism are extremely low within the P. falciparum genome compared to that of closely related, ape infecting species of Plasmodium (including P. praefalciparum). This suggests that the origin of P. falciparum in humans is recent, as a single P. praefalciparum strain became capable of infecting humans. The genetic information of Plasmodium falciparum
has signaled a recent expansion that coincides with the agricultural
revolution. It is likely that the development of extensive agriculture
increased mosquito population densities by giving rise to more breeding
sites, which may have triggered the evolution and expansion of Plasmodium falciparum.
Structure
Blood smear from a P. falciparum culture
(K1 strain - asexual forms) - several red blood cells have ring stages
inside them. Close to the center is a schizont and on the left a
trophozoite.
P. falciparum does not have a fixed structure but undergoes
continuous change during the course of its life cycle. A sporozoite is
spindle-shaped and 10-15 μm long. In the liver it grows into an ovoid
schizont of 30-70 μm in diameter. Each schizont produces merozoites,
each of which is roughly 1.5 μm in length and 1 μm in diameter. In the
erythrocyte the merozoite form a ring-like structure, becoming a
trophozoite. A trophozoites feed on the haemoglobin and forms a granular
pigment called haemozoin. Unlike those of other Plasmodium species, the gametocytes of P. falciparum
are elongated and crescent-shaped, by which they are sometimes
identified. A mature gametocyte is 8-12 μm long and 3-6 μm wide. The
ookinete is also elongated measuring about 18-24 μm. An oocyst is
rounded and can grow up to 80 μm in diameter.
Microscopic examination of a blood film reveals only early (ring-form)
trophozoites and gametocytes that are in the peripheral blood. Mature
trophozoites or schizonts in peripheral blood smears, as these are
usually sequestered in the tissues. On occasion, faint, comma-shaped,
red dots are seen on the erythrocyte surface. These dots are Maurer's cleft and are secretory organelles that produce proteins and enzymes essential for nutrient uptake and immune evasion processes.
The apical complex, which is actually a combination of
organelles, is an important structure. It contains secretory organelles
called rhoptries and micronemes, which are vital for mobility, adhesion,
host cell invasion, and parasitophorous vacuole formation. As an apicomplexan, it harbours a plastid, an apicoplast, similar to plant chloroplasts, which they probably acquired by engulfing (or being invaded by) a eukaryotic alga and retaining the algal plastid as a distinctive organelle encased within four membranes. The apicoplast is involved in the synthesis of lipids
and several other compounds and provides an attractive drug target.
During the asexual blood stage of infection, an essential function of
the apicoplast is to produce the isoprenoid precursors isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP) via the MEP (non-mevalonate) pathway .
Genome
In 1995 the Malaria Genome Project was set up to sequence the genome of P. falciparum. The genome of its mitochondrion was reported in 1995, that of the nonphotosynthetic plastid known as the apicoplast in 1996, and the sequence of the first nuclear chromosome (chromosome 2) in 1998. The sequence of chromosome 3 was reported in 1999 and the entire genome was reported on 3 October 2002.
The roughly 24-megabase genome is extremely AT-rich (about 80%) and is
organised into 14 chromosomes. Just over 5,300 genes were described.
Many genes involved in antigenic variation are located in the
subtelomeric regions of the chromosomes. These are divided into the var, rif, and stevor families. Within the genome, there exist 59 var, 149 rif, and 28 stevor
genes, along with multiple pseudogenes and truncations. It is
estimated that 551, or roughly 10%, of the predicted nuclear-encoded proteins are targeted to the apicoplast, while 4.7% of the proteome is targeted to the mitochondria.
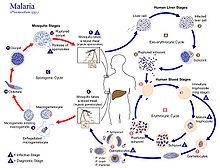
Life cycle
Humans
are the intermediate hosts in which asexual reproduction occurs, and
female anopheline mosquitos are the definitive hosts harbouring the
sexual reproduction stage.
In humans
Life cycle of Plasmodium
Infection in humans begins with the bite of an infected female Anopheles mosquito. Out of about 460 species of Anopheles mosquito, more than 70 species transmit falciparum malaria. Anopheles gambiae is one of the best known and most prevalent vectors, particularly in Africa.
The infective stage called sporozoites
released from the salivary glands through the proboscis of the mosquito
enter the bloodstream during feeding. The mosquito saliva contains
antihemostatic and anti-inflammatory enzymes that disrupt blood clotting
and inhibit the pain reaction. Typically, each infected bite contains
20-200 sporozoites.
The immune system clears the sporozoites from the circulation within 30
minutes. But a few escape and quickly invade liver cells (hepatocytes).
The sporozoites move in the blood stream by gliding, which is driven by
motor made up of proteins actin and myosin beneath their plasma
membrane.
Liver stage or exo-erythrocytic schizogony
Entering the hepatocytes, the parasite loses its apical complex and surface coat, and transforms into a trophozoite. Within the parasitophorous vacuole of the hepatocyte, it undergoes 13-14 rounds of mitosis and meiosis which produce a syncytial cell (coenocyte)
called a schizont. This process is called schizogony. A schizont
contains tens of thousands of nuclei. From the surface of the schizont,
tens of thousands of haploid (1n) daughter cells called merozoites
emerge. The liver stage can produce up to 90,000 merozoites, which are eventually released into the bloodstream in parasite-filled vesicles called merosomes.
Blood stage or erythrocytic schizogony
Merozoites use the apicomplexan invasion organelles (apical complex,
pellicle and surface coat) to recognize and enter the host erythrocyte
(red blood cell). The parasite first binds to the erythrocyte in a
random orientation. It then reorients such that the apical complex is in
proximity to the erythrocyte membrane. The parasite forms a
parasitophorous vacuole, to allow for its development inside the erythrocyte.
This infection cycle occurs in a highly synchronous fashion, with
roughly all of the parasites throughout the blood in the same stage of
development. This precise clocking mechanism has been shown to be
dependent on the human host's own circadian rhythm.
Within the erythrocyte, the parasite metabolism depends on the digestion of hemoglobin. The clinical symptoms of malaria such as fever, anemia, and neurological disorder are produced during the blood stage.
The parasite can also alter the morphology of the erythrocyte,
causing knobs on the erythrocyte membrane. Infected erythrocytes are
often sequestered in various human tissues or organs, such as the heart,
liver and brain. This is caused by parasite-derived cell surface
proteins being present on the erythrocyte membrane, and it is these
proteins that bind to receptors on human cells. Sequestration in the
brain causes cerebral malaria, a very severe form of the disease, which
increases the victim's likelihood of death.
Trophozoite
After
invading the erythrocyte, the parasite loses its specific invasion
organelles (apical complex and surface coat) and de-differentiates into a
round trophozoite located within a parasitophorous vacuole. The young
trophozoite (or "ring" stage, because of its morphology on stained blood
films) grows substantially before undergoing schizogony.
Schizont
At the schizont stage, the parasite replicates its DNA multiple times and multiple mitotic divisions occur asynchronously. Each schizont forms 16-18 merozoites.
The red blood cells are ruptured by the merozoites. The liberated
merozoites invade fresh erythrocytes. A free merozoite is in the
bloodstream for roughly 60 seconds before it enters another erythrocyte.
The duration of each blood stage is approximately 48 hours. This
gives rise to the characteristic clinical manifestations of falciparum
malaria, such as fever and chills, corresponding to the synchronous
rupture of the infected erythrocytes.
Gametocyte
Some merozoites differentiate into sexual forms, male and female gametocytes.
These gametocytes take roughly 7–15 days to reach full maturity,
through the process called gametocytogenesis. These are then taken up by
a female Anopheles mosquito during a blood meal.
Incubation period
The time of appearance of the symptoms from infection (called incubation period) is shortest for P. falciparum among Plasmodium species. An average incubation period is 11 days,
but may range from 9 to 30 days. In isolated cases, prolonged
incubation period as long as 2, 3 or even 8 years have been recorded. Pregnancy and co-infection with HIV are important conditions for delayed symptoms. Parasites can be detected from blood samples by the 10th day after infection (pre-patent period).
In mosquitoes
Within
the mosquito midgut, the female gamete maturation process entails
slight morphological changes, becoming more enlarged and spherical. The
male gametocyte undergoes a rapid nuclear division within 15 minutes,
producing eight flagellated microgametes by a process called exflagellation. The flagellated microgamete fertilizes the female macrogamete to produce a diploid cell called a zygote. The zygote then develops into an ookinete. The ookinete is a motile cell, capable of invading other organs of the mosquito. It traverses the peritrophic membrane of the mosquito midgut and crosses the midgut epithelium. Once through the epithelium, the ookinete enters the basal lamina, and settles to an immotile oocyst. For several days, the oocyst undergoes 10 to 11 rounds of cell division to create a syncytial cell (sporoblast)
containing thousands of nuclei. Meiosis takes place inside the
sporoblast to produce over 3,000 haploid daughter cells called
sporozoites on the surface of the mother cell. Immature sporozoites break through the oocyst wall into the haemolymph. They migrate to the mosquito salivary glands where they undergo further development and become infective to humans.
Interaction with human immune system
Immune response
A single anopheline mosquito can transmit hundreds of P. falciparum sporozoites in a single bite under experimental conditions. But in nature the number is generally less than 80.
The sporozoites do not enter the blood stream directly and remain in
the skin tissue for 2 to 3 hours. About 15–20% sporozoites enter the
lymphatic system where they activate dendritic cells, which send them for destruction by T lymphocytes (CD8+ T cells). At 48 hours after infection, Plasmodium-specific CD8+ T cells can be detected in the lymph nodes connected to the skin cells. Most of the sporozites remaining in the skin tissue are subsequently killed by the innate immune system. The sporozoite glycoprotein specifically activates mast cells. The mast cells then produce signalling molecules such as TNFα and MIP-2, which activate cell eaters (professional phagocytes) such as neutrophils and macrophages.
Only a small number (0.5-5%) of sporozoites enter the blood
stream into the liver. In the liver, the activated CD8+ T cells from the
lymph bind the sporozoites through the circumsporozoite protein (CSP). Antigen presentation
by dendritic cells in the skin tissue to T cells is also a crucial
process. From this stage onward the parasites produce different proteins
that help in suppressing communication of the immune cells.
Even at the height of the infection when RBCs are ruptured, the immune
signals are not strong enough to activate macrophages or natural killer cells.
Immune system evasion
Although P. falciparum
is easily recognized by human immune system while in the bloodstream,
it evades immunity by producing over 2,000 cell membrane antigens The initial infective stage sporozoites produce circumsporozoite protein (CSP), which binds to hepatocytes. Binding to and entry into the hepatocytes is aided by another protein, thrombospondin-related anonymous protein (TRAP).
TRAP and other secretory proteins (including sporozoite microneme
protein essential for cell traversal 1, SPECT1 and SPECT2) from
microneme allow the sporozoite to move through the blood, avoiding
immune cells and penetrating hepatocytes.
During erythrocyte invasion, merozoites release merozoite cap
protein-1 (MCP1), apical membrane antigen 1 (AMA1), erythrocyte-binding
antigens (EBA), myosin A tail domain interacting protein (MTIP), and
merozoite surface proteins (MSPs). Of these MSPs, MSP1 and MSP2 are primarily responsible for avoiding immune cells. The virulence of P. falciparum
is mediated by erythrocyte membrane proteins, which are produced by the
schizonts and trophozoites inside the erythrocytes and are displayed on
the erythrocyte membrane. PfEMP1 is the most important, capable of acting as both an antigen and an adhesion molecule.
Pathogenesis
The clinical symptoms of falciparum malaria are produced by the
rupture of schizont and destruction of erythrocytes. Most of the
patients experience fever (>92% of cases), chills (79%), headaches (70%), and sweating (64%). Dizziness, malaise, muscle pain, abdominal pain, nausea, vomiting, mild diarrhea, and dry cough are also generally associated. High heartrate, jaundice, pallor, orthostatic hypotension, enlarged liver, and enlarged spleen are also diagnosed.
P. falciparum works via sequestration, a distinctive property not shared by few other Plasmodiae.
The mature schizonts change the surface properties of infected
erythrocytes, causing them to stick to blood vessel walls
(cytoadherence). This leads to obstruction of the microcirculation and
results in dysfunction of multiple organs, such as the brain in cerebral malaria.
P. falciparum is responsible for (almost) all severe human
illnesses and deaths due to malaria, in a condition called complicated
or severe malaria. Complicated malaria occurs more commonly in children
under age 5, and sometimes in pregnant women (a condition specifically called pregnancy-associated malaria).
Women become susceptible to severe malaria during their first
pregnancy. Susceptibility to severe malaria is reduced in subsequent
pregnancies due to increased antibody levels against variant surface antigens that appear on infected erythrocytes. But increased immunity in mother increases susceptibility to malaria in newborn babies.
Distribution and epidemiology
The Z(T) normalized index of temperature suitability for P. falciparum displayed by week across an average year.
P. falciparum is found in all continents except Europe. According to the WHO World Malaria Report 2019, 228 million people suffered from malaria in 2018, a slight decrease from 231 million in 2017. 405,000 people died from it.
The infection is most prevalent in Africa, where 94% of malaria deaths
occur. Children under five years of age are most affected and 61% of
malaria deaths occurred in this age group. 80% of the infection is found
in Sub-Saharan Africa, 7% in the South-East Asia, and 2% in the Eastern
Mediterranean. Nigeria has the highest incidence with 27% of the total
global cases. Outside Africa, India has the highest incidence with 4.5%
of the global burden. Europe is regarded as a malaria-free region.
Historically, the parasite and its disease had been most well known in
Europe. But medical programmes, such as insecticide spraying, drug
therapy and environmental engineering since the early 20th century
resulted in complete eradication in the 1970s. It is estimated that approximately 2.4 billion people are at constant risk of infection.
Treatment
History
In 1640, Huan del Vego first employed the tincture of the cinchona bark for treating malaria; the native Indians of Peru and Ecuador had been using it even earlier for treating fevers. Thompson (1650) introduced this "Jesuits' bark" to England. Its first recorded use there was by John Metford of Northampton in 1656. Morton (1696) presented the first detailed description of the clinical picture of malaria and of its treatment with cinchona. Gize (1816) studied the extraction of crystalline quinine from the cinchona bark and Pelletier and Caventou (1820) in France extracted pure quinine alkaloids, which they named quinine and cinchonine.
The total synthesis of quinine was achieved by American chemists R.B.
Woodward and W.E. Doering in 1944. Woodward received the Nobel Prize in
Chemistry in 1965.
Attempts to make synthetic antimalarials began in 1891. Atabrine, developed in 1933, was used widely throughout the Pacific in World War II, but was unpopular because of its adverse effects.[68] In the late 1930s, the Germans developed chloroquine, which went into use in the North African campaigns. Creating a secret military project called Project 523, Mao Zedong encouraged Chinese scientists to find new antimalarials after seeing the casualties in the Vietnam War. Tu Youyou discovered artemisinin in the 1970s from sweet wormwood (Artemisia annua).
This drug became known to Western scientists in the late 1980s and
early 1990s and is now a standard treatment. Tu won the Nobel Prize in
Physiology or Medicine in 2015.
Uncomplicated malaria
According to WHO guidelines 2010, artemisinin-based combination therapies (ACTs) are the recommended first-line antimalarial treatments for uncomplicated malaria caused by P. falciparum. WHO recommends combinations such as artemether/lumefantrine, artesunate/amodiaquine, artesunate/mefloquine, artesunate/sulfadoxine-pyrimethamine, and dihydroartemisinin/piperaquine.
The choice of ACT is based on the level of resistance to the
constituents in the combination. Artemisinin and its derivatives are not
appropriate for monotherapy. As second-line antimalarial treatment,
when initial treatment does not work, an alternative ACT known to be
effective in the region is recommended, such as artesunate plus
tetracycline or doxycycline or clindamycin, and quinine
plus tetracycline or doxycycline or clindamycin. Any of these
combinations is to be given for 7 days. For pregnant women, the
recommended first-line treatment during the first trimester is quinine plus clindamycin for 7 days. Artesunate plus clindamycin for 7 days is indicated if this treatment fails. For travellers returning to nonendemic countries, atovaquone/proguanil, artemether/lumefantrineany and quinine plus doxycycline or clindamycin are recommended.
Severe malaria
For adults, intravenous (IV) or intramuscular (IM) artesunate is recommended. Quinine is an acceptable alternative if parenteral artesunate is not available.
For children, especially in the malaria-endemic areas of Africa,
artesunate IV or IM, quinine (IV infusion or divided IM injection), and
artemether IM are recommended.
Parenteral antimalarials should be administered for a minimum of
24 hours, irrespective of the patient's ability to tolerate oral
medication earlier. Thereafter, complete treatment is recommended including complete course of ACT or quinine plus clindamycin or doxycycline.
Vaccination
RTS,S is the only candidate as malaria vaccine to have gone through clinical trials.
Analysis of the results of the phase III trial (conducted between 2011
and 2016) revealed a rather low efficacy (20-39% depending on age, with
up to 50% in 5–17-month aged babies), indicating that the vaccine will
not lead to full protection and eradication.
Cancer
The International Agency for Research on Cancer (IARC) has classified malaria due to P. falciparum as Group 2A carcinogen, meaning that the parasite is probably a cancer-causing agent in humans. Its association with a blood cell (lymphocyte) cancer called Burkitt's lymphoma is established. Burkit's lymphoma was discovered by Denis Burkitt
in 1958 from African children, and he later speculated that the cancer
was likely due to certain infectious diseases. In 1964, a virus, later
called Epstein–Barr virus
(EBV) after the discoverers, was identified from the cancer cells. The
virus was subsequently proved to be the direct cancer agent, and is now
classified as Group 1 carcinogen.
In 1989, it was realised that EBV requires other infections such as
with malaria to cause lymphocyte transformation. It was reported that
the incidence of Burkitt's lymphoma decreased with effective treatment
of malaria over several years. The actual role played by P. falciparum
remained unclear for the next two-and-half decades. EBV had been known
to induce lymphocytes to become cancerous using its viral proteins
(antigens such as EBNA-1, EBNA-2, LMP-1, and LMP2A). From 2014, it became clear that P. falciparum contributes to the development of the lymphoma. P. falciparum-infected erythrocytes directly bind to B lymphocytes through the CIDR1α domain of PfEMP1. This binding activates toll-like receptors (TLR7 and TLR10) causing continuous activation of lymphocytes to undergo proliferation and differentiation into plasma cells, thereby increasing the secretion of IgM and cytokines. This in turn activates an enzyme called activation-induced cytidine deaminase (AID), which tends to cause mutation in the DNA (by double-strand break) of an EBV-infected lymphocytes. The damaged DNA undergoes uncontrolled replication, thus making the cell cancerous.
Influence on the human genome
The high mortality and morbidity caused by P. falciparum has placed great selective pressure on the human genome. Several genetic factors provide some resistance to Plasmodium infection, including sickle cell trait, thalassaemia traits, glucose-6-phosphate dehydrogenase deficiency, and the absence of Duffy antigens on red blood cells. E. A. Beet, a doctor working in Southern Rhodesia (now Zimbabwe) had observed in 1948 that sickle-cell disease was related to a lower rate of malaria infections. This suggestion was reiterated by J. B. S. Haldane in 1948, who suggested that thalassaemia might provide similar protection. This hypothesis has since been confirmed and extended to hemoglobin E, hemoglobin C and Hemoglobin S.