
Modern 3
tesla clinical MRI scanner.
From Wikipedia
The
physics of magnetic resonance imaging (MRI) involves the interaction of
biological tissue with
electromagnetic fields. MRI is a
medical imaging technique used in radiology to investigate the anatomy and physiology of the body. The human body is
largely composed of water molecules, each containing two
hydrogen nuclei, or
protons. When inside the
magnetic field (B
0) of the scanner, the
magnetic moments of these protons align with the direction of the field.
A
radio frequency
pulse is then applied, causing the protons to alter their magnetization
alignment relative to the field. In response to the force bringing them
back to their equilibrium orientation, the protons undergo a rotating
motion (
precession), much like a spin wheel under the effect of gravity. These changes in magnetization alignment cause a changing
magnetic flux,
which yields a changing voltage in receiver coils to give the signal.
The frequency at which a proton or group of protons in a
voxel
resonates depends on the strength of the local magnetic field around
the proton or group of protons. By applying additional magnetic fields
(gradients) that vary linearly over space, specific slices to be imaged
can be selected, and an image is obtained by taking the 2-D
Fourier transform of the spatial frequencies of the signal (a.k.a.,
k-space). Due to the magnetic
Lorentz force from B
0
on the current flowing in the gradient coils, the gradient coils will
try to move. The knocking sounds heard during an MRI scan are the result
of the gradient coils trying to move against the constraint of the
concrete or epoxy in which they are secured. These sounds can be very
unnerving to the patient, particularly given the tight space in which
the patient lays. This behaviour of MRI scanners can be described in
terms of a fully coupled acousto-magneto-mechanical system.
[1] Solutions to such systems can provide useful insight for design engineers.
Diseased tissue, such as
tumors,
can be detected because the protons in different tissues return to
their equilibrium state at different rates (i.e., they have different
relaxation
times). By changing the parameters on the scanner this effect is used
to create contrast between different types of body tissue.
Contrast agents may be injected
intravenously to enhance the appearance of
blood vessels,
tumors or
inflammation. Contrast agents may also be directly injected into a joint in the case of arthrograms, MRI images of joints. Unlike
CT, MRI uses no
ionizing radiation and is generally a very safe procedure. Patients with some metal implants,
cochlear implants,
and cardiac pacemakers are prevented from having an MRI scan due to
effects of the strong magnetic field and powerful radio frequency
pulses unless the device they carry is labeled MR-Conditional.
[2]
MRI is used to image every part of the body, and is particularly
useful for neurological conditions, for disorders of the muscles and
joints, for evaluating tumors, and for showing abnormalities in the
heart and blood vessels.
Nuclear magnetism
Subatomic particles have the
quantum mechanical property of
spin.
[3] Certain nuclei such as
1H (
protons),
2H,
3He,
23Na or
31P, have a non–zero spin and therefore a
magnetic moment. In the case of the so-called
spin-1⁄2 nuclei, such as
1H, there are two spin states, sometimes referred to as
up and
down. Nuclei such as
12C have no unpaired neutrons or protons, and no net spin; however, the isotope
13C does.
When these spins are placed in a strong external magnetic field they
precess around an axis along the direction of the field. Protons align in two energy
eigenstates (the
Zeeman effect): one low-energy and one high-energy, which are separated by a very small splitting energy.
Resonance and relaxation
In the static magnetic fields commonly used in MRI, the energy
difference between the nuclear spin states corresponds to a
radio-frequency
photon. Resonant absorption of energy by the protons due to an external oscillating magnetic field will occur at the
Larmor frequency for the particular nucleus.
The spin of the proton has two states. The net longitudinal
magnetization in thermodynamical equilibrium is due to a tiny excess of
protons in the lower energy state. This gives a net polarization that is
parallel to the external field. Application of a
radio frequency
(RF) pulse can tip this net polarization vector sideways (with, i.e., a
so-called 90° pulse), or even reverse it (with a so-called 180° pulse).
The recovery of longitudinal magnetization is called longitudinal or
T1 relaxation and occurs exponentially with a time constant
T1. The loss of phase coherence in the transverse plane is called transverse or
T2 relaxation.
T1 is thus associated with the
enthalpy of the spin system, or the number of nuclei with parallel versus anti-parallel spin.
T2 on the other hand is associated with the
entropy of the system, or the number of nuclei in phase.
When the radio frequency pulse is turned off, the transverse vector
component produces an oscillating magnetic field which induces a small
current in the receiver coil. This signal is called the
free induction decay (FID). In an idealized
nuclear magnetic resonance experiment, the FID decays approximately exponentially with a time constant
T2.
However, in practical MRI there are small differences in the static
magnetic field at different spatial locations ("inhomogeneities") that
cause the Larmor frequency to vary across the body. This creates
destructive interference, which shortens the FID. The time constant for
the observed decay of the FID is called the
T*
2 relaxation time, and is always shorter than
T2. At the same time, the longitudinal magnetization starts to recover exponentially with a time constant
T1 which is much larger than
T2 (see below).
In MRI, the static magnetic field is caused to vary across the body
(by using a field gradient), so that different spatial locations become
associated with different precession frequencies. Usually these field
gradients are pulsed, and it is the almost infinite variety of RF and
gradient pulse sequences that gives MRI its versatility. Application of
field gradient destroys the FID signal, but this can be recovered and
measured by a refocusing gradient (to create a so-called "gradient
echo"), or by a radio frequency pulse (to create a so-called
"spin-echo"). The whole process can be repeated when some
T1-relaxation has occurred and the thermal equilibrium of the spins has been more or less restored. The
repetition time (TR) is the time between two successive excitations of the same slice.
[4]
Typically, in soft tissues
T1 is around one second while
T2 and
T*
2
are a few tens of milliseconds. However, these values can vary widely
between different tissues, as well as between different external
magnetic fields. This behavior is one factor giving MRI its tremendous
soft tissue contrast.
MRI contrast agents, such as those containing
Gadolinium(III) work by altering (shortening) the relaxation parameters, especially
T1.
Imaging
Imaging schemes
A number of schemes have been devised for combining field gradients and radio frequency excitation to create an image:
- 2D or 3D reconstruction from projections, such as in computed tomography.
- Building the image point-by-point or line-by-line.
- Gradients in the RF field rather than the static field.
Although each of these schemes is occasionally used in
specialist applications, the majority of MR Images today are created
either by the two-dimensional
Fourier transform
(2DFT) technique with slice selection, or by the three-dimensional
Fourier transform (3DFT) technique. Another name for 2DFT is spin-warp.
What follows here is a description of the 2DFT technique with slice
selection.
The 3DFT technique is rather similar except that there is no slice
selection and phase-encoding is performed in two separate directions.
Echo-planar imaging
Another scheme which is sometimes used, especially in brain scanning
or where images are needed very rapidly, is called echo-planar imaging
(EPI):
[5]
In this case, each RF excitation is followed by a train of gradient
echoes with different spatial encoding. Multiplexed-EPI is even faster,
e.g., for whole brain
fMRI or
diffusion MRI.
[6]
Image contrast and contrast enhancement
Image
contrast
is created by differences in the strength of the NMR signal recovered
from different locations within the sample. This depends upon the
relative density of excited nuclei (usually water protons), on
differences in
relaxation times (
T1,
T2, and
T*
2) of those nuclei after the pulse sequence, and often on other parameters discussed under
specialized MR scans.
Contrast in most MR images is actually a mixture of all these effects,
but careful design of the imaging pulse sequence allows one contrast
mechanism to be emphasized while the others are minimized. The ability
to choose different contrast mechanisms gives MRI tremendous
flexibility. In the brain,
T1-weighting causes the nerve connections of
white matter to appear white, and the congregations of neurons of
gray matter to appear gray, while
cerebrospinal fluid (CSF) appears dark. The contrast of white matter, gray matter and cerebrospinal fluid is reversed using
T2 or
T*
2
imaging, whereas proton-density-weighted imaging provides little
contrast in healthy subjects. Additionally, functional parameters such
as
cerebral blood flow (CBF), cerebral blood volume (CBV) or
blood oxygenation can affect
T1,
T2, and
T*
2 and so can be encoded with suitable pulse sequences.
In some situations it is not possible to generate enough image contrast to adequately show the
anatomy or
pathology of interest by adjusting the imaging parameters alone, in which case a
contrast agent may be administered. This can be as simple as
water, taken orally, for imaging the stomach and small bowel. However, most
contrast agents used in MRI are selected for their specific magnetic properties. Most commonly, a
paramagnetic contrast agent (usually a
gadolinium compound
[7][8]) is given. Gadolinium-enhanced tissues and fluids appear extremely bright on
T1-weighted
images. This provides high sensitivity for detection of vascular
tissues (e.g., tumors) and permits assessment of brain perfusion (e.g.,
in stroke). There have been concerns raised recently regarding the
toxicity of gadolinium-based contrast agents and their impact on persons
with impaired kidney function. (See
Safety/
Contrast agents below.)
More recently,
superparamagnetic contrast agents, e.g.,
iron oxide nanoparticles,
[9][10] have become available. These agents appear very dark on
T*
2-weighted images and may be used for liver imaging, as normal
liver
tissue retains the agent, but abnormal areas (e.g., scars, tumors) do
not. They can also be taken orally, to improve visualization of the
gastrointestinal tract, and to prevent water in the gastrointestinal tract from obscuring other organs (e.g., the
pancreas).
Diamagnetic agents such as
barium sulfate have also been studied for potential use in the
gastrointestinal tract, but are less frequently used.
k-space
In 1983, Ljunggren
[11] and Twieg
[12] independently introduced the
k-space
formalism, a technique that proved invaluable in unifying different MR
imaging techniques. They showed that the demodulated MR signal
S(
t) generated by freely precessing nuclear spins in the presence of a linear magnetic field gradient
G equals the Fourier transform of the effective spin density. Mathematically:

where:

In other words, as time progresses the signal traces out a trajectory in
k-space
with the velocity vector of the trajectory proportional to the vector
of the applied magnetic field gradient. By the term
effective spin density we mean the true spin density

corrected for the effects of
T1 preparation,
T2
decay, dephasing due to field inhomogeneity, flow, diffusion, etc. and
any other phenomena that affect that amount of transverse magnetization
available to induce signal in the RF probe or its phase with respect to
the receiving coil' s electromagnetic field.
From the basic
k-space formula, it follows immediately that we reconstruct an image

simply by taking the
inverse Fourier transform of the sampled data, viz.

Using the
k-space formalism, a number of seemingly
complex ideas became simple. For example, it becomes very easy to
understand the role of phase encoding (the so-called spin-warp method).
In a standard spin echo or gradient echo scan, where the readout (or
view) gradient is constant (e.g.,
G), a single line of
k-space is scanned per RF excitation. When the phase encoding gradient is zero, the line scanned is the
kx
axis. When a non-zero phase-encoding pulse is added in between the RF
excitation and the commencement of the readout gradient, this line moves
up or down in
k-space, i.e., we scan the line
ky = constant.
The
k-space formalism also makes it very easy to compare different scanning techniques. In single-shot
EPI, all of
k-space is scanned in a single shot, following either a sinusoidal or zig-zag trajectory. Since alternating lines of
k-space
are scanned in opposite directions, this must be taken into account in
the reconstruction. Multi-shot EPI and fast spin echo techniques acquire
only part of
k-space per excitation. In each shot, a different interleaved segment is acquired, and the shots are repeated until
k-space is sufficiently well-covered. Since the data at the center of
k-space represent lower spatial frequencies than the data at the edges of
k-space, the
TE value for the center of
k-space determines the image's
T2 contrast.
The importance of the center of
k-space in determining image
contrast can be exploited in more advanced imaging techniques. One such
technique is spiral acquisition—a rotating magnetic field gradient is
applied, causing the trajectory in
k-space to spiral out from the center to the edge. Due to
T2 and
T*
2 decay the signal is greatest at the start of the acquisition, hence acquiring the center of
k-space first improves
contrast to noise ratio (CNR) when compared to conventional zig-zag acquisitions, especially in the presence of rapid movement.
Since

and

are conjugate variables (with respect to the Fourier transform) we can use the
Nyquist theorem to show that the step in
k-space
determines the field of view of the image (maximum frequency that is
correctly sampled) and the maximum value of k sampled determines the
resolution; i.e.,

(These relationships apply to each axis independently.)
Example of a pulse sequence

Simplified timing diagram for two-dimensional-Fourier-transform (2DFT) Spin Echo (SE) pulse sequence
In the timing diagram, the horizontal axis represents time. The
vertical axis represents: (top row) amplitude of radio frequency pulses;
(middle rows) amplitudes of the three orthogonal magnetic field
gradient pulses; and (bottom row) receiver analog-to-digital converter
(ADC). Radio frequencies are transmitted at the Larmor frequency of the
nuclide to be imaged. For example, for
1H in a magnetic field of 1
T, a frequency of 42.5781
MHz would be employed. The three field gradients are labeled
GX (typically corresponding to a patient's left-to-right direction and colored red in diagram),
GY (typically corresponding to a patient's front-to-back direction and colored green in diagram), and
GZ
(typically corresponding to a patient's head-to-toe direction and
colored blue in diagram). Where negative-going gradient pulses are
shown, they represent reversal of the gradient direction, i.e.,
right-to-left, back-to-front or toe-to-head. For human scanning,
gradient strengths of 1–100 mT/m are employed: Higher gradient strengths
permit better resolution and faster imaging. The pulse sequence shown
here would produce a transverse (axial) image.
The first part of the pulse sequence, SS, achieves "slice selection". A shaped pulse (shown here with a
sinc modulation) causes a 90°
nutation
of longitudinal nuclear magnetization within a slab, or slice, creating
transverse magnetization. The second part of the pulse sequence, PE,
imparts a phase shift upon the slice-selected nuclear magnetization,
varying with its location in the Y direction. The third part of the
pulse sequence, another slice selection (of the same slice) uses another
shaped pulse to cause a 180° rotation of transverse nuclear
magnetization within the slice. This transverse magnetisation refocuses
to form a spin echo at a time
TE. During the spin
echo, a frequency-encoding (FE) or readout gradient is applied, making
the resonant frequency of the nuclear magnetization vary with its
location in the X direction. The signal is sampled
nFE times by the ADC during this period, as represented by the vertical lines. Typically
nFE of between 128 and 512 samples are taken.
The longitudinal magnetisation is then allowed to recover somewhat and after a time
TR the whole sequence is repeated
nPE
times, but with the phase-encoding gradient incremented (indicated by
the horizontal hatching in the green gradient block). Typically
nPE of between 128 and 512 repetitions are made.
The negative-going lobes in
GX and
GZ are imposed to ensure that, at time
TE (the spin echo maximum), phase only encodes spatial location in the Y direction.
Typically
TE is between 5 ms and 100 ms, while
TR is between 100 ms and 2000 ms.
After the two-dimensional matrix (typical dimension between 128 × 128 and 512 × 512) has been acquired, producing the so-called
k-space
data, a two-dimensional inverse Fourier transform is performed to
provide the familiar MR image. Either the magnitude or phase of the
Fourier transform can be taken, the former being far more common.
Overview of main sequences
This table does not include
uncommon and experimental sequences.
| Group |
Sequence |
Abbr. |
Physics |
Main clinical distinctions |
Example |
|---|
| Spin echo |
T1 weighted |
T1 |
Measuring spin–lattice relaxation by using a short repetition time (TR) and echo time (TE) |
Standard foundation and comparison for other sequences. |
 |
| T2 weighted |
T2 |
Measuring spin–spin relaxation by using long TR and TE times. |
Standard foundation and comparison for other sequences. |
 |
| Proton density weighted |
PD |
Long TR (to reduce T1) and short TE (to minimize T2)[15] |
Joint disease and injury.[16]
|
 |
| Gradient echo |
Steady-state free precession |
SSFP |
Maintenance of a steady, residual transverse magnetisation over successive cycles.[18] |
Creation of cardiac MRI videos (pictured).[18] |
 |
| Inversion recovery |
Short tau inversion recovery |
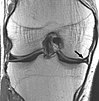
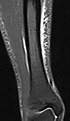
STIR |
Fat suppression by setting an inversion time where the signal of fat is zero.[19] |
High signal in edema, such as in more severe stress fracture.[20] Shin splints pictured: |
 |
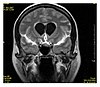
| Fluid attenuated inversion recovery |
FLAIR |
Fluid suppression by setting an inversion time that nulls fluids. |
High signal in lacunar infarction, multiple sclerosis (MS) plaques, subarachnoid haemorrhage and meningitis (pictured).[21] |
 |
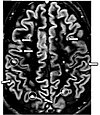
| Double inversion recovery |
DIR |
Simultaneous suppression of cerebrospinal fluid and white matter by two inversion times.[22] |
High signal of multiple sclerosis plaques (pictured).[22] |
 |
| Diffusion weighted (DWI) |
Conventional |
DWI |
Measure of Brownian motion of water molecules.[23] |
High signal within minutes of cerebral infarction (pictured).[24] |
 |
| Apparent diffusion coefficient |
ADC |
Reduced T2 weighting by taking multiple conventional DWI images with
different DWI weighting, and the change corresponds to diffusion.[25] |
Low signal minutes after cerebral infarction (pictured).[26] |
 |
| Diffusion tensor |
DTI |
Mainly tractography (pictured) by an overall greater Brownian motion of water molecules in the directions of nerve fibers.[27] |
|
 |
| Perfusion weighted (PWI) |
Dynamic susceptibility contrast |
DSC |
Gadolinium contrast is injected, and rapid repeated imaging (generally gradient-echo echo-planar T2 weighted) quantifies susceptibility-induced signal loss.[29] |
In cerebral infarction, the infarcted core and the penumbra have decreased perfusion (pictured).[30] |
 |
| Dynamic contrast enhanced |
DCE |
Measuring shortening of the spin–lattice relaxation (T1) induced by a gadolinium contrast bolus.[31] |
| Arterial spin labelling |
ASL |
Magnetic labeling of arterial blood below the imaging slab, which subsequently enters the region of interest.[32] It does not need gadolinium contrast.[33] |
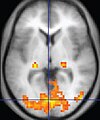
| Functional MRI (fMRI) |
Blood-oxygen-level dependent imaging |
BOLD |
Changes in oxygen saturation-dependent magnetism of hemoglobin reflects tissue activity.[34] |
Localizing highly active brain areas before surgery.[35] |
 |
| Magnetic resonance angiography (MRA) and venography |
Time-of-flight |
TOF |
Blood entering the imaged area is not yet magnetically saturated, giving it a much higher signal when using short echo time and flow compensation. |
Detection of aneurysm, stenosis or dissection.[36] |
 |
| Phase-contrast MRA |
PC-MRA |
Two gradients with equal magnitude but opposite direction are used
to encode a phase shift, which is proportional to the velocity of spins.[37] |
Detection of aneurysm, stenosis or dissection (pictured).[36] |

(VIPR) |
| Susceptibility weighted |
SWI |
Sensitive for blood and calcium, by a fully flow compensated, long echo, gradient recalled echo (GRE) pulse sequence to exploit magnetic susceptibility differences between tissues. |
Detecting small amounts of hemorrhage (diffuse axonal injury pictured) or calcium.[38] |
 |
MRI scanner
Construction and operation

Schematic of construction of a cylindrical superconducting MR scanner
The major components of an MRI scanner are: the main magnet, which
polarizes the sample, the shim coils for correcting inhomogeneities in
the main magnetic field, the gradient system which is used to localize
the MR signal and the RF system, which excites the sample and detects
the resulting NMR signal. The whole system is controlled by one or more
computers.
Magnet
The magnet is the largest and most expensive component of the
scanner, and the remainder of the scanner is built around it. The
strength of the magnet is measured in
teslas (T).
Clinical magnets generally have a field strength in the range
0.1–3.0 T, with research systems available up to 9.4 T for human use and
21 T for animal systems.
[39] In the United States, field strengths up to 4 T have been approved by the FDA for clinical use.
[40]
Just as important as the strength of the main magnet is its
precision. The straightness of the magnetic lines within the center (or,
as it is technically known, the iso-center) of the magnet needs to be
near-perfect. This is known as homogeneity. Fluctuations
(inhomogeneities in the field strength) within the scan region should be
less than three parts per million (3 ppm). Three types of magnets have
been used:
- Permanent magnet: Conventional magnets made from ferromagnetic materials (e.g., steel alloys containing rare-earth elements such as neodymium)
can be used to provide the static magnetic field. A permanent magnet
that is powerful enough to be used in an MRI will be extremely large and
bulky; they can weigh over 100 tonnes. Permanent magnet MRIs are very
inexpensive to maintain; this cannot be said of the other types of MRI
magnets, but there are significant drawbacks to using permanent magnets.
They are only capable of achieving weak field strengths compared to
other MRI magnets (usually less than 0.4 T) and they are of limited
precision and stability. Permanent magnets also present special safety
issues; since their magnetic fields cannot be "turned off,"
ferromagnetic objects are virtually impossible to remove from them once
they come into direct contact. Permanent magnets also require special
care when they are being brought to their site of installation.
- Resistive electromagnet: A solenoid
wound from copper wire is an alternative to a permanent magnet. An
advantage is low initial cost, but field strength and stability are
limited. The electromagnet requires considerable electrical energy
during operation which can make it expensive to operate. This design is
essentially obsolete.
- Superconducting electromagnet: When a niobium-titanium or niobium-tin alloy is cooled by liquid helium to 4 K (−269 °C, −452 °F) it becomes a superconductor,
losing resistance to flow of electric current. An electromagnet
constructed with superconductors can have extremely high field
strengths, with very high stability. The construction of such magnets is
extremely costly, and the cryogenic helium is expensive and difficult
to handle. However, despite their cost, helium cooled superconducting
magnets are the most common type found in MRI scanners today.
Most superconducting magnets have their coils of superconductive wire immersed in liquid helium, inside a vessel called a
cryostat. Despite thermal insulation, sometimes including a second cryostat containing
liquid nitrogen,
ambient heat causes the helium to slowly boil off. Such magnets,
therefore, require regular topping-up with liquid helium. Generally a
cryocooler,
also known as a coldhead, is used to recondense some helium vapor back
into the liquid helium bath. Several manufacturers now offer
'cryogenless' scanners, where instead of being immersed in liquid helium
the magnet wire is cooled directly by a cryocooler.
[41]
Magnets are available in a variety of shapes. However, permanent
magnets are most frequently 'C' shaped, and superconducting magnets most
frequently cylindrical. However, C-shaped superconducting magnets and
box-shaped permanent magnets have also been used.
Magnetic field strength is an important factor in determining image quality. Higher magnetic fields increase
signal-to-noise ratio,
permitting higher resolution or faster scanning. However, higher field
strengths require more costly magnets with higher maintenance costs, and
have increased safety concerns. A field strength of 1.0–1.5 T is a good
compromise between cost and performance for general medical use.
However, for certain specialist uses (e.g., brain imaging) higher field
strengths are desirable, with some hospitals now using 3.0 T scanners.

FID signal from a badly shimmed sample has a complex envelope.

FID signal from a well shimmed sample, showing a pure exponential decay.
Shims
When the MR scanner is placed in the hospital or clinic, its main
magnetic field is far from being homogeneous enough to be used for
scanning. That is why before doing fine tuning of the field using a
sample, the magnetic field of the magnet must be measured and
shimmed.
After a sample is placed into the scanner, the main magnetic field is distorted by
susceptibility
boundaries within that sample, causing signal dropout (regions showing
no signal) and spatial distortions in acquired images. For humans or
animals the effect is particularly pronounced at air-tissue boundaries
such as the
sinuses (due to
paramagnetic
oxygen in air) making, for example, the frontal lobes of the brain
difficult to image. To restore field homogeneity a set of shim coils is
included in the scanner. These are resistive coils, usually at room
temperature, capable of producing field corrections distributed as
several orders of
spherical harmonics.
[42]
After placing the sample in the scanner, the
B0 field is 'shimmed' by adjusting currents in the shim coils. Field homogeneity is measured by examining an
FID
signal in the absence of field gradients. The FID from a poorly shimmed
sample will show a complex decay envelope, often with many humps. Shim
currents are then adjusted to produce a large amplitude exponentially
decaying FID, indicating a homogeneous
B0 field. The process is usually automated.
[43]
Gradients
Gradient coils are used to spatially encode the positions of protons
by varying the magnetic field linearly across the imaging volume. The
Larmor frequency will then vary as a function of position in the
x,
y and
z-axes.
Gradient coils are usually resistive electromagnets powered by
sophisticated amplifiers which permit rapid and precise adjustments to
their field strength and direction. Typical gradient systems are capable
of producing gradients from 20–100 mT/m (i.e., in a 1.5 T magnet, when a
maximal
z-axis gradient is applied, the field strength may be 1.45 T at one end of a 1 m long bore and 1.55 T at the other
[44]).
It is the magnetic gradients that determine the plane of
imaging—because the orthogonal gradients can be combined freely, any
plane can be selected for imaging.
Scan speed is dependent on performance of the gradient system.
Stronger gradients allow for faster imaging, or for higher resolution;
similarly, gradient systems capable of faster switching can also permit
faster scanning. However, gradient performance is limited by safety
concerns over nerve stimulation.
Some important characteristics of gradient amplifiers and gradient
coils are slew rate and gradient strength. As mentioned earlier, a
gradient coil will create an additional, linearly varying magnetic field
that adds or subtracts from the main magnetic field. This additional
magnetic field will have components in all 3 directions, viz.
x,
y and
z; however, only the component along the magnetic field (usually called the
z-axis, hence denoted
Gz)
is useful for imaging. Along any given axis, the gradient will add to
the magnetic field on one side of the zero position and subtract from it
on the other side. Since the additional field is a gradient, it has
units of
gauss
per centimeter or millitesla per meter (mT/m). High performance
gradient coils used in MRI are typically capable of producing a gradient
magnetic field of approximate 30 mT/m or higher for a 1.5 T MRI. The
slew rate of a gradient system is a measure of how quickly the gradients
can be ramped on or off. Typical higher performance gradients have a
slew rate of up to 100–200 T·m
−1·s
−1. The slew
rate depends both on the gradient coil (it takes more time to ramp up or
down a large coil than a small coil) and on the performance of the
gradient amplifier (it takes a lot of voltage to overcome the inductance
of the coil) and has significant influence on image quality.
Radio frequency system
The radio frequency (RF) transmission system consists of an RF synthesizer, power amplifier and
transmitting coil.
That coil is usually built into the body of the scanner. The power of
the transmitter is variable, but high-end whole-body scanners may have a
peak output power of up to 35 kW,
[45] and be capable of sustaining average power of 1 kW. Although these
electromagnetic fields are in the RF range of tens of
megahertz (often in the
shortwave radio portion of the
electromagnetic spectrum) at powers usually exceeding the highest powers used by
amateur radio,
there is very little RF interference produced by the MRI machine. The
reason for this, is that the MRI is not a radio transmitter. The RF
frequency
electromagnetic field produced in the "transmitting coil" is a magnetic
near-field with very little associated changing
electric field
component (such as all conventional radio wave transmissions have).
Thus, the high-powered electromagnetic field produced in the MRI
transmitter coil does not produce much
electromagnetic radiation
at its RF frequency, and the power is confined to the coil space and
not radiated as "radio waves." Thus, the transmitting coil is a good EM
field transmitter at radio frequency, but a poor EM
radiation transmitter at radio frequency.
The receiver consists of the coil, pre-amplifier and signal processing system. The RF
electromagnetic radiation
produced by nuclear relaxation inside the subject is true EM radiation
(radio waves), and these leave the subject as RF radiation, but they are
of such low power as to also not cause appreciable RF interference that
can be picked up by nearby radio tuners (in addition, MRI scanners are
generally situated in metal mesh lined rooms which act as
Faraday cages.)
While it is possible to scan using the integrated coil for RF
transmission and MR signal reception, if a small region is being imaged,
then better image quality (i.e., higher signal-to-noise ratio) is
obtained by using a close-fitting smaller coil. A variety of coils are
available which fit closely around parts of the body such as the head,
knee, wrist, breast, or internally, e.g., the rectum.
A recent development in MRI technology has been the development of sophisticated multi-element phased array
[46]
coils which are capable of acquiring multiple channels of data in
parallel. This 'parallel imaging' technique uses unique acquisition
schemes that allow for accelerated imaging, by replacing some of the
spatial coding originating from the magnetic gradients with the spatial
sensitivity of the different coil elements. However, the increased
acceleration also reduces the signal-to-noise ratio and can create
residual artifacts in the image reconstruction. Two frequently used
parallel acquisition and reconstruction schemes are known as SENSE
[47] and GRAPPA.
[48] A detailed review of parallel imaging techniques can be found here:
[49]































