From Wikipedia, the free encyclopedia
| Yeast
|
|---|

|
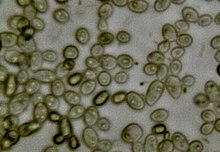
| Yeast of the species Saccharomyces cerevisiae
|

|
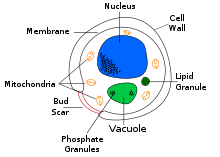
| Cross-sectional labelled diagram of a typical yeast cell
|
| Scientific classification
|
| Domain:
|
|
| Kingdom:
|
|
| Phyla and Subphyla
|
|
|
Yeasts are
eukaryotic single-celled microorganisms classified as members of the
fungus kingdom. The first yeast originated hundreds of millions of years ago, and 1,500
species are currently identified. They are estimated to constitute 1% of all described fungal species. Yeasts are unicellular organisms which evolved from multicellular ancestors, with some species having the ability to develop
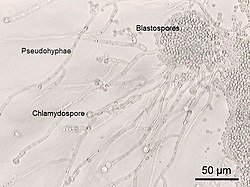
multicellular characteristics by forming strings of connected budding cells known as
pseudohyphae or false hyphae. Yeast sizes vary greatly, depending on species and environment, typically measuring 3–4
µm in
diameter, although some yeasts can grow to 40 µm in size. Most yeasts reproduce
asexually by
mitosis, and many do so by the asymmetric division process known as
budding.
Yeasts, with their single-celled growth habit, can be contrasted with
molds, which grow
hyphae. Fungal species that can take both forms (depending on temperature or other conditions) are called
dimorphic fungi ("dimorphic" means "having two forms").
By
fermentation, the yeast species
Saccharomyces cerevisiae converts
carbohydrates to
carbon dioxide and
alcohols – for thousands of years the carbon dioxide has been used in
baking and the alcohol in alcoholic beverages. It is also a centrally important
model organism in modern
cell biology research, and is one of the most thoroughly researched
eukaryotic
microorganisms. Researchers have used it to gather information about
the biology of the eukaryotic cell and ultimately human biology. Other species of yeasts, such as
Candida albicans, are
opportunistic pathogens and can cause
infections in humans. Yeasts have recently been used to generate electricity in
microbial fuel cells, and produce
ethanol for the
biofuel industry.
Yeasts do not form a single
taxonomic or
phylogenetic grouping. The term "yeast" is often taken as a
synonym for
Saccharomyces cerevisiae, but the phylogenetic diversity of yeasts is shown by their placement in two separate
phyla: the
Ascomycota and the
Basidiomycota. The budding yeasts ("true yeasts") are classified in the
order Saccharomycetales, within the phylum Ascomycota.
History
The word "yeast" comes from
Old English gist,
gyst, and from the
Indo-European root
yes-, meaning "boil", "foam", or "bubble".
Yeast microbes are probably one of the earliest domesticated organisms.
Archaeologists digging in Egyptian ruins found early grinding stones
and baking chambers for yeast-raised bread, as well as drawings of
4,000-year-old bakeries and breweries. In 1680,
Dutch naturalist
Anton van Leeuwenhoek first
microscopically observed yeast, but at the time did not consider them to be
living organisms, but rather globular structures as researchers were doubtful whether yeasts were algae or fungi.
Theodor Schwann recognized them as fungi in 1837.
In 1857, French microbiologist
Louis Pasteur showed that by bubbling oxygen into the yeast broth,
cell growth could be increased, but fermentation was inhibited – an observation later called the "
Pasteur effect". In the paper "
Mémoire sur la fermentation alcoolique," Pasteur proved that alcoholic fermentation was conducted by living yeasts and not by a chemical catalyst.
By the late 18th century two yeast strains used in brewing had been identified:
Saccharomyces cerevisiae (top-fermenting yeast) and
S. carlsbergensis (bottom-fermenting yeast).
S. cerevisiae has been sold commercially by the Dutch for bread-making since 1780; while, around 1800, the Germans started producing
S. cerevisiae in the form of cream. In 1825, a method was developed to remove the liquid so the yeast could be prepared as solid blocks. The industrial production of yeast blocks was enhanced by the introduction of the
filter press
in 1867. In 1872, Baron Max de Springer developed a manufacturing
process to create granulated yeast, a technique that was used until the
first World War.
In the United States, naturally occurring airborne yeasts were used
almost exclusively until commercial yeast was marketed at the
Centennial Exposition in 1876 in Philadelphia, where
Charles L. Fleischmann exhibited the product and a process to use it, as well as serving the resultant baked bread.
The
mechanical refrigerator (first patented in the 1850s in Europe) liberated
brewers and
winemakers from seasonal constraints for the first time and allowed them to exit cellars and other earthen environments. For
John Molson, who made his livelihood in
Montreal
prior to the development of the fridge, the brewing season lasted from
September through to May. The same seasonal restrictions formerly
governed the
distiller's art.
Nutrition and growth
Yeasts are
chemoorganotrophs, as they use
organic compounds as a source of energy and do not require sunlight to grow. Carbon is obtained mostly from
hexose sugars, such as
glucose and
fructose, or disaccharides such as
sucrose and
maltose. Some species can metabolize
pentose sugars such as ribose, alcohols, and
organic acids. Yeast species either require oxygen for aerobic
cellular respiration (
obligate aerobes) or are anaerobic, but also have aerobic methods of energy production (
facultative anaerobes). Unlike
bacteria, no known yeast species grow only anaerobically (
obligate anaerobes). Most yeasts grow best in a neutral or slightly acidic pH environment.
Yeasts vary in regard to the temperature range in which they grow best. For example,
Leucosporidium frigidum grows at −2 to 20 °C (28 to 68 °F),
Saccharomyces telluris at 5 to 35 °C (41 to 95 °F), and
Candida slooffi at 28 to 45 °C (82 to 113 °F). The cells can survive freezing under certain conditions, with viability decreasing over time.
In general, yeasts are grown in the laboratory on solid
growth media or in liquid
broths. Common media used for the cultivation of yeasts include
potato dextrose agar or
potato dextrose broth, Wallerstein Laboratories nutrient
agar, yeast
peptone dextrose agar, and yeast mould agar or broth. Home brewers who cultivate yeast frequently use dried
malt extract and agar as a solid growth medium. The
antibiotic cycloheximide is sometimes added to yeast growth media to inhibit the growth of
Saccharomyces yeasts and select for wild/indigenous yeast species. This will change the yeast process.
The appearance of a white, thready yeast, commonly known as kahm
yeast, is often a byproduct of the lactofermentation (or pickling) of
certain vegetables, usually the result of exposure to air. Although
harmless, it can give pickled vegetables a bad flavor and must be
removed regularly during fermentation.
Ecology
Yeasts are very common in the environment, and are often isolated
from sugar-rich materials. Examples include naturally occurring yeasts
on the skins of fruits and berries (such as grapes, apples, or
peaches), and exudates from plants (such as plant saps or cacti). Some yeasts are found in association with soil and insects. The ecological function and
biodiversity of yeasts are relatively unknown compared to those of other
microorganisms. Yeasts, including
Candida albicans,
Rhodotorula rubra,
Torulopsis and
Trichosporon cutaneum, have been found living in between people's toes as part of their
skin flora. Yeasts are also present in the
gut flora of mammals and some insects and even deep-sea environments host an array of yeasts.
An Indian study of seven
bee species and 9 plant species found 45 species from 16 genera colonise the
nectaries of flowers and honey stomachs of bees. Most were members of the genus
Candida; the most common species in honey stomachs was
Dekkera intermedia and in flower nectaries,
Candida blankii. Yeast colonising nectaries of the
stinking hellebore
have been found to raise the temperature of the flower, which may aid
in attracting pollinators by increasing the evaporation of
volatile organic compounds. A
black yeast has been recorded as a partner in a complex relationship between
ants, their
mutualistic fungus, a fungal
parasite
of the fungus and a bacterium that kills the parasite. The yeast has a
negative effect on the bacteria that normally produce antibiotics to
kill the parasite, so may affect the ants' health by allowing the
parasite to spread.
Certain strains of some species of yeasts produce proteins called
yeast killer toxins that allow them to eliminate competing strains.
This can cause problems for winemaking but could potentially also be
used to advantage by using killer toxin-producing strains to make the
wine. Yeast killer toxins may also have medical applications in treating
yeast infections (see "Pathogenic yeasts" section below).
Marine yeasts, defined as the yeasts that are isolated from
marine environments, are able to grow better on a medium prepared using
seawater rather than freshwater. The first marine yeasts were isolated by Bernhard Fischer in 1894 from the Atlantic Ocean, and those were identified as
Torula sp. and
Mycoderma sp.
Following this discovery, various other marine yeasts have been
isolated from around the world from different sources, including
seawater, seaweeds, marine fish and mammals.
Among these isolates, some marine yeasts originated from terrestrial
habitats (grouped as facultative marine yeast), which were brought to
and survived in marine environments. The other marine yeasts were
grouped as obligate or indigenous marine yeasts, which confine to marine
habitats. However, no sufficient evidence has been found to explain the indispensability of seawater for obligate marine yeasts.
It has been reported that marine yeasts are able to produce many
bioactive substances, such as amino acids, glucans, glutathione, toxins,
enzymes, phytase and vitamins with potential application in the food,
pharmaceutical, cosmetic and chemical industries as well as for marine
culture and environmental protection. Marine yeast was successfully used to produce bioethanol using seawater-based media which will potentially reduce the
water footprint of bioethanol.
Reproduction
The yeast cell's life cycle:
- Budding
- Conjugation
- Spore
Yeasts, like all fungi, may have
asexual and
sexual reproductive cycles. The most common mode of vegetative growth in yeast is asexual reproduction by
budding, where a small bud (also known as a
bleb or daughter cell) is formed on the parent cell. The
nucleus
of the parent cell splits into a daughter nucleus and migrates into the
daughter cell. The bud then continues to grow until it separates from
the parent cell, forming a new cell. The daughter cell produced during the budding process is generally smaller than the mother cell. Some yeasts, including
Schizosaccharomyces pombe, reproduce by
fission instead of budding, and thereby creating two identically sized daughter cells.
In general, under high-stress conditions such as
nutrient starvation,
haploid cells will die; under the same conditions, however,
diploid cells can undergo sporulation, entering sexual reproduction (
meiosis) and producing a variety of haploid
spores, which can go on to
mate (conjugate), reforming the diploid.
The haploid fission yeast
Schizosaccharomyces pombe is a
facultative sexual microorganism that can undergo mating when nutrients are limiting. Exposure of
S. pombe
to hydrogen peroxide, an agent that causes oxidative stress leading to
oxidative DNA damage, strongly induces mating and the formation of
meiotic spores. The budding yeast
Saccharomyces cerevisiae
reproduces by mitosis as diploid cells when nutrients are abundant, but
when starved, this yeast undergoes meiosis to form haploid spores. Haploid cells may then reproduce asexually by mitosis. Katz Ezov et al. presented evidence that in natural
S. cerevisiae
populations clonal reproduction and selfing (in the form of intratetrad
mating) predominate. In nature, mating of haploid cells to form diploid
cells is most often between members of the same clonal population and
out-crossing is uncommon. Analysis of the ancestry of natural
S. cerevisiae strains led to the conclusion that out-crossing occurs only about once every 50,000 cell divisions.
These observations suggest that the possible long-term benefits of
outcrossing (e.g. generation of diversity) are likely to be insufficient
for generally maintaining sex from one generation to the next. Rather, a short-term benefit, such as recombinational repair during meiosis, may be the key to the maintenance of sex in
S. cerevisiae.
Uses
Alcoholic beverages
Alcoholic beverages are defined as
beverages that contain
ethanol (C
2H
5OH). This ethanol is almost always produced by
fermentation – the
metabolism of
carbohydrates by certain species of yeasts under anaerobic or low-oxygen conditions. Beverages such as mead, wine, beer, or
distilled spirits
all use yeast at some stage of their production. A distilled beverage
is a beverage containing ethanol that has been purified by
distillation.
Carbohydrate-containing plant material is fermented by yeast, producing
a dilute solution of ethanol in the process. Spirits such as
whiskey and
rum
are prepared by distilling these dilute solutions of ethanol.
Components other than ethanol are collected in the condensate, including
water,
esters, and other alcohols, which (in addition to that provided by the oak in which it may be aged) account for the
flavour of the beverage.
Beer
Yeast ring used by Swedish farmhouse brewers in the 19th century to preserve yeast between brewing sessions.
Brewing yeasts may be classed as "top-cropping" (or "top-fermenting") and "bottom-cropping" (or "bottom-fermenting"). Top-cropping yeasts are so called because they form a foam at the top of the
wort during fermentation. An example of a top-cropping yeast is
Saccharomyces cerevisiae, sometimes called an "ale yeast". Bottom-cropping yeasts are typically used to produce
lager-type beers, though they can also produce
ale-type beers. These yeasts ferment well at low temperatures. An example of bottom-cropping yeast is
Saccharomyces pastorianus, formerly known as
S. carlsbergensis.
Decades ago, taxonomists reclassified
S. carlsbergensis (uvarum) as a member of
S. cerevisiae, noting that the only distinct difference between the two is metabolic. Lager strains of
S. cerevisiae secrete an enzyme called melibiase, allowing them to hydrolyse
melibiose, a
disaccharide, into more fermentable
monosaccharides.
Top- and bottom-cropping and cold- and warm-fermenting distinctions are
largely generalizations used by laypersons to communicate to the
general public.
The most common top-cropping brewer's yeast,
S. cerevisiae, is the same species as the common baking yeast. Brewer's yeast is also very rich in
essential minerals and the
B vitamins (except B
12).
However, baking and brewing yeasts typically belong to different
strains, cultivated to favour different characteristics: baking yeast
strains are more aggressive, to carbonate
dough
in the shortest amount of time possible; brewing yeast strains act more
slowly but tend to produce fewer off-flavours and tolerate higher
alcohol concentrations (with some strains, up to 22%).
Dekkera/Brettanomyces is a genus of yeast known for its important role in the production of '
lambic' and specialty
sour ales, along with the secondary conditioning of a particular Belgian
Trappist beer. The taxonomy of the genus
Brettanomyces
has been debated since its early discovery and has seen many
reclassifications over the years. Early classification was based on a
few species that reproduced asexually (anamorph form) through multipolar
budding. Shortly after, the formation of ascospores was observed and the genus
Dekkera, which reproduces sexually (teleomorph form), was introduced as part of the taxonomy. The current taxonomy includes five species within the genera of
Dekkera/Brettanomyces. Those are the anamorphs
Brettanomyces bruxellensis,
Brettanomyces anomalus,
Brettanomyces custersianus,
Brettanomyces naardenensis, and
Brettanomyces nanus, with teleomorphs existing for the first two species,
Dekkera bruxellensis and
Dekkera anomala. The distinction between
Dekkera and
Brettanomyces
is arguable, with Oelofse et al. (2008) citing Loureiro and
Malfeito-Ferreira from 2006 when they affirmed that current molecular
DNA detection techniques have uncovered no variance between the anamorph
and teleomorph states. Over the past decade,
Brettanomyces spp.
have seen an increasing use in the craft-brewing sector of the industry,
with a handful of breweries having produced beers that were primarily
fermented with pure cultures of
Brettanomyces spp. This has
occurred out of experimentation, as very little information exists
regarding pure culture fermentative capabilities and the aromatic
compounds produced by various strains.
Dekkera/
Brettanomyces
spp. have been the subjects of numerous studies conducted over the past
century, although a majority of the recent research has focused on
enhancing the knowledge of the wine industry. Recent research on eight
Brettanomyces
strains available in the brewing industry focused on strain-specific
fermentations and identified the major compounds produced during pure
culture anaerobic fermentation in wort.
Wine
Yeast is used in
winemaking, where it converts the sugars present (
glucose and
fructose) into
grape juice (
must) into ethanol. Yeast is normally already present on grape skins.
Fermentation can be done with this endogenous "wild yeast",
but this procedure gives unpredictable results, which depend upon the
exact types of yeast species present. For this reason, a pure yeast
culture is usually added to the must; this yeast quickly dominates the
fermentation. The wild yeasts are repressed, which ensures a reliable
and predictable fermentation.
Most added wine yeasts are strains of S. cerevisiae, though not all strains of the species are suitable. Different S. cerevisiae
yeast strains have differing physiological and fermentative properties,
therefore the actual strain of yeast selected can have a direct impact
on the finished wine.
Significant research has been undertaken into the development of novel
wine yeast strains that produce atypical flavour profiles or increased
complexity in wines.
The growth of some yeasts, such as
Zygosaccharomyces and
Brettanomyces, in wine can result in
wine faults and subsequent spoilage.
Brettanomyces produces an array of
metabolites when growing in wine, some of which are volatile
phenolic compounds. Together, these compounds are often referred to as "
Brettanomyces character", and are often described as "
antiseptic" or "barnyard" type aromas.
Brettanomyces is a significant contributor to wine faults within the wine industry.
Baking
Yeast, the most common one being
S. cerevisiae, is used in baking as a
leavening agent, where it converts the
food/fermentable sugars present in dough into the gas
carbon dioxide.
This causes the dough to expand or rise as gas forms pockets or
bubbles. When the dough is baked, the yeast dies and the air pockets
"set", giving the baked product a soft and spongy texture. The use of
potatoes, water from potato boiling,
eggs,
or sugar in a bread dough accelerates the growth of yeasts. Most yeasts
used in baking are of the same species common in alcoholic
fermentation. In addition,
Saccharomyces exiguus (also known as
S. minor),
a wild yeast found on plants, fruits, and grains, is occasionally used
for baking. In breadmaking, the yeast initially respires aerobically,
producing carbon dioxide and water. When the oxygen is depleted,
fermentation begins, producing ethanol as a waste product; however, this evaporates during baking.
A block of compressed fresh yeast
It is not known when yeast was first used to bake bread. The first records that show this use came from
Ancient Egypt.
Researchers speculate a mixture of flour meal and water was left longer
than usual on a warm day and the yeasts that occur in natural
contaminants of the
flour caused it to ferment before baking. The resulting bread would have been lighter and tastier than the normal flat, hard cake.
Active dried yeast, a granulated form in which yeast is commercially sold
Today, there are several retailers of baker's yeast; one of the earlier developments in North America is
Fleischmann's Yeast, in 1868. During World War II, Fleischmann's developed a
granulated active dry yeast which did not require refrigeration, had a longer
shelf life
than fresh yeast, and rose twice as fast. Baker's yeast is also sold as
a fresh yeast compressed into a square "cake". This form perishes
quickly, so must be used soon after production. A weak solution of water
and sugar can be used to determine whether yeast is expired. In the
solution, active yeast will foam and bubble as it ferments the sugar
into ethanol and carbon dioxide. Some recipes refer to this as
proofing the yeast, as it "proves" (tests) the viability of the yeast before the other ingredients are added. When a
sourdough starter is used, flour and water are added instead of sugar; this is referred to as proofing the
sponge.
When yeast is used for making bread, it is mixed with
flour, salt, and warm water or milk. The dough is
kneaded
until it is smooth, and then left to rise, sometimes until it has
doubled in size. The dough is then shaped into loaves. Some bread doughs
are knocked back after one rising and left to rise again (this is
called
dough proofing)
and then baked. A longer rising time gives a better flavour, but the
yeast can fail to raise the bread in the final stages if it is left for
too long initially.
Bioremediation
Industrial ethanol production
The ability of yeast to convert sugar into ethanol has been harnessed by the biotechnology industry to produce
ethanol fuel. The process starts by milling a feedstock, such as
sugar cane,
field corn, or other
cereal grains, and then adding dilute
sulfuric acid, or fungal alpha
amylase
enzymes, to break down the starches into complex sugars. A glucoamylase
is then added to break the complex sugars down into simple sugars.
After this, yeasts are added to convert the simple sugars to ethanol,
which is then distilled off to obtain ethanol up to 96% in purity.
Saccharomyces yeasts have been
genetically engineered to ferment
xylose, one of the major fermentable sugars present in
cellulosic biomasses, such as agriculture residues, paper wastes, and wood chips. Such a development means ethanol can be efficiently produced from more inexpensive feedstocks, making
cellulosic ethanol fuel a more competitively priced alternative to gasoline fuels.
Nonalcoholic beverages
Yeast and bacteria in kombucha at 400×
A number of sweet
carbonated beverages
can be produced by the same methods as beer, except the fermentation is
stopped sooner, producing carbon dioxide, but only trace amounts of
alcohol, leaving a significant amount of residual sugar in the drink.
- Root beer, originally made by Native Americans, commercialized in the United States by Charles Elmer Hires and especially popular during Prohibition
- Kvass, a fermented drink made from rye, popular in Eastern Europe. It has a recognizable, but low alcoholic content.
- Kombucha, a fermented sweetened tea. Yeast in symbiosis with acetic acid bacteria is used in its preparation. Species of yeasts found in the tea can vary, and may include: Brettanomyces bruxellensis, Candida stellata, Schizosaccharomyces pombe, Torulaspora delbrueckii and Zygosaccharomyces bailii. Also popular in Eastern Europe and some former Soviet republics under the name chajnyj grib (Russian: Чайный гриб), which means "tea mushroom".
- Kefir and kumis are made by fermenting milk with yeast and bacteria.
- Mauby (Spanish: mabí), made by fermenting sugar with the wild yeasts naturally present on the bark of the Colubrina elliptica tree, popular in the Caribbean
Nutritional supplements
Yeast is used in nutritional supplements, especially those marketed to
vegans. It is often referred to as "
nutritional yeast" when sold as a dietary supplement. Nutritional yeast is a deactivated yeast, usually
S. cerevisiae. It is naturally low in fat and
sodium as well as an excellent source of protein and vitamins, especially most
B-complex vitamins (contrary to some claims, it contains little or no vitamin B
12), as well as other minerals and
cofactors required for growth. Some brands of nutritional yeast, though not all, are fortified with
vitamin B12, which is produced separately by
bacteria.
In 1920, the
Fleischmann Yeast Company
began to promote yeast cakes in a "Yeast for Health" campaign. They
initially emphasized yeast as a source of vitamins, good for skin and
digestion. Their later advertising claimed a much broader range of
health benefits, and was censured as misleading by the
Federal Trade Commission. The
fad for yeast cakes lasted until the late 1930s.
Nutritional yeast has a nutty, cheesy flavor and is often used as
an ingredient in cheese substitutes. Another popular use is as a
topping for popcorn. It can also be used in mashed and fried potatoes,
as well as in
scrambled eggs. It comes in the form of flakes, or as a yellow powder similar in texture to
cornmeal.
In Australia, it is sometimes sold as "savoury yeast flakes". Though
"nutritional yeast" usually refers to commercial products, inadequately
fed prisoners have used "home-grown" yeast to prevent vitamin
deficiency.
Probiotics
Aquarium hobby
Yeast is often used by
aquarium hobbyists to generate carbon dioxide (CO
2) to nourish plants in
planted aquaria. CO
2 levels from yeast are more difficult to regulate than those from pressurized CO
2 systems. However, the low cost of yeast makes it a widely used alternative.
Marmite and Vegemite are dark in colour
Yeast extract is the common name for various forms of processed yeast products that are used as
food additives or
flavours. They are often used in the same way that
monosodium glutamate (MSG) is used and, like MSG, often contain free
glutamic acid. The general method for making yeast extract for food products such as
Vegemite and
Marmite
on a commercial scale is to add salt to a suspension of yeast, making
the solution hypertonic, which leads to the cells' shrivelling up. This
triggers
autolysis, wherein the yeast's
digestive enzymes break their own
proteins
down into simpler compounds, a process of self-destruction. The dying
yeast cells are then heated to complete their breakdown, after which the
husks (yeast with thick cell walls that would give poor texture) are
separated. Yeast autolysates are used in
Vegemite and
Promite (Australia);
Marmite (the United Kingdom); the unrelated
Marmite (New Zealand);
Vitam-R (Germany); and
Cenovis (
Switzerland).
Scientific research
Diagram showing a yeast cell
Several yeasts, in particular
S. cerevisiae and
S. pombe, have been widely used in genetics and cell biology, largely because they are simple
eukaryotic cells, serving as a model for all eukaryotes, including humans, for the study of fundamental cellular processes such as the
cell cycle,
DNA replication,
recombination,
cell division,
and metabolism. Also, yeasts are easily manipulated and cultured in the
laboratory, which has allowed for the development of powerful standard
techniques, such as
yeast two-hybrid,
synthetic genetic array analysis, and
tetrad analysis. Many proteins important in human biology were first discovered by studying their
homologues in yeast; these proteins include
cell cycle proteins,
signaling proteins, and protein-processing
enzymes.
On 24 April 1996,
S. cerevisiae was announced to be the first eukaryote to have its
genome, consisting of 12 million
base pairs, fully sequenced as part of the
Genome Project.
At the time, it was the most complex organism to have its full genome
sequenced, and the work seven years and the involvement of more than 100
laboratories to accomplish. The second yeast species to have its genome sequenced was
Schizosaccharomyces pombe, which was completed in 2002.
It was the sixth eukaryotic genome sequenced and consists of
13.8 million base pairs. As of 2014, over 50 yeast species have had
their genomes sequenced and published.
Genomic and functional gene annotation of the two major yeast models can be accessed via their respective
model organism databases: SGD and PomBase.
Genetically engineered biofactories
Various yeast species have been genetically engineered to efficiently produce various drugs, a technique called
metabolic engineering.
S. cerevisiae
is easy to genetically engineer; its physiology, metabolism and
genetics are well known, and it is amenable for use in harsh industrial
conditions. A wide variety of chemical in different classes can be
produced by engineered yeast, including
phenolics,
isoprenoids,
alkaloids, and
polyketides. About 20% of
biopharmaceuticals are produced in
S. cerevisiae, including
insulin,
vaccines for
hepatitis, and
human serum albumin.
Pathogenic yeasts
Yeasts of the genus
Candida, another group of opportunistic pathogens, cause
oral and
vaginal infections in humans, known as
candidiasis.
Candida is commonly found as a
commensal yeast in the
mucous membranes
of humans and other warm-blooded animals. However, sometimes these same
strains can become pathogenic. The yeast cells sprout a
hyphal outgrowth, which locally penetrates the
mucosal membrane, causing irritation and shedding of the tissues. The pathogenic yeasts of candidiasis in probable descending order of
virulence for humans are:
C. albicans,
C. tropicalis,
C. stellatoidea,
C. glabrata,
C. krusei,
C. parapsilosis,
C. guilliermondii,
C. viswanathii,
C. lusitaniae, and
Rhodotorula mucilaginosa.
Candida glabrata is the second most common
Candida pathogen after
C. albicans, causing infections of the
urogenital tract, and of the
bloodstream (
candidemia).
Food spoilage
Yeasts are able to grow in foods with a low pH (5.0 or lower) and in
the presence of sugars, organic acids, and other easily metabolized
carbon sources.
During their growth, yeasts metabolize some food components and produce
metabolic end products. This causes the physical, chemical, and
sensible properties of a food to change, and the food is spoiled.
The growth of yeast within food products is often seen on their
surfaces, as in cheeses or meats, or by the fermentation of sugars in
beverages, such as juices, and semiliquid products, such as
syrups and
jams. The yeast of the genus
Zygosaccharomyces have had a long history as spoilage yeasts within the
food industry. This is mainly because these species can grow in the presence of high sucrose, ethanol,
acetic acid,
sorbic acid,
benzoic acid, and
sulphur dioxide concentrations, representing some of the commonly used
food preservation methods.
Methylene blue is used to test for the presence of live yeast cells. In
oenology, the major spoilage yeast is
Brettanomyces bruxellensis.
Symbiosis
An Indian study of seven
bee species and 9 plant species found 45 yeast species from 16 genera colonise the
nectaries of flowers and honey stomachs of bees. Most were members of the genus
Candida; the most common species in honey bee stomachs was
Dekkera intermedia, while the most common species colonising flower nectaries was
Candida blankii. Although the mechanics are not fully understood, it was found that
A. indica flowers more if
C. blankii are present.