From Wikipedia, the free encyclopedia
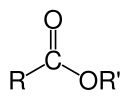
In chemistry, esters are chemical compounds derived from an acid (organic or inorganic) in which at least one -OH (hydroxyl) group is replaced by an -O-alkyl (alkoxy) group.[1] Usually, esters are derived from a carboxylic acid and an alcohol. Esters comprise most naturally occurring fats and oils.[dubious ] An important case are glycerides, which are fatty acid esters of glycerol. Esters with low molecular weight are commonly used as fragrances and found in essential oils and pheromones. Phosphoesters form the backbone of DNA molecules. Nitrate esters, such as nitroglycerin, are known for their explosive properties, while polyesters are important plastics, with monomers linked by ester moieties.
Nomenclature
Etymology
The word 'ester' was coined in 1848 by German chemist Leopold Gmelin,[2] probably as a contraction of the German Essigäther, "acetic ether".IUPAC nomenclature
Ester names are derived from the parent alcohol and the parent acid, where the latter may be organic or inorganic. Esters derived from the simplest carboxylic acids are commonly named according to the more traditional, so-called "trivial names" e.g. as formate, acetate, propionate, and butyrate, as opposed to the IUPAC nomenclature methanoate, ethanoate, propanoate and butanoate. Esters derived from more complex carboxylic acids are, on the other hand, more frequently named using the systematic IUPAC name, based on the name for the acid followed by the suffix -oate. For example the ester hexyl octanoate, also known under the trivial name hexyl caprylate, has the formula CH3(CH2)6CO2(CH2)5CH3.
Ethyl acetate derived from an alcohol (blue) and an acyl group (yellow) derived from a carboxylic acid.
The chemical formulas of organic esters usually take the form RCO2R', where R and R' are the hydrocarbon parts of the carboxylic acid and the alcohol, respectively. For example butyl acetate (systematically butyl ethanoate), derived from butanol and acetic acid (systematically ethanoic acid) would be written CH3CO2C4H9. Alternative presentations are common including BuOAc and CH3COOC4H9.
Cyclic esters are called lactones, regardless of whether they are derived from an organic or an inorganic acid. One example of a (organic) lactone is gamma-valerolactone.
Orthoesters
An uncommon class of organic esters are the orthoesters, which have the formula RC(OR')3. Triethylorthoformate (HC(OC2H5)3) is derived, in terms of its name (but not its synthesis) from orthoformic acid (HC(OH)3) and ethanol.Inorganic esters
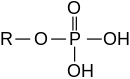
Esters can also be derived from an inorganic acid and an alcohol. Thus, the nomenclature extends to inorganic oxo acids, e.g. phosphoric acid, sulfuric acid, nitric acid and boric acid. For example, triphenyl phosphate is the ester derived from phosphoric acid and phenol. Organic carbonates are derived from carbonic acid; for example, ethylene carbonate is derived from carbonic acid and ethylene glycol.
Structure and bonding
Esters contain a carbonyl center, which gives rise to 120 °C-C-O and O-C-O angles. Unlike amides, esters are structurally flexible functional groups because rotation about the C-O-C bonds has a low barrier. Their flexibility and low polarity is manifested in their physical properties; they tend to be less rigid (lower melting point) and more volatile (lower boiling point) than the corresponding amides.[3] The pKa of the alpha-hydrogens on esters is around 25.[4]Physical properties and characterization
Esters are more polar than ethers but less polar than alcohols. They participate in hydrogen bonds as hydrogen-bond acceptors, but cannot act as hydrogen-bond donors, unlike their parent alcohols. This ability to participate in hydrogen bonding confers some water-solubility. Because of their lack of hydrogen-bond-donating ability, esters do not self-associate. Consequently esters are more volatile than carboxylic acids of similar molecular weight.[3]Characterization and analysis
Esters are generally identified by gas chromatography, taking advantage of their volatility. IR spectra for esters feature an intense sharp band in the range 1730–1750 cm−1 assigned to νC=O. This peak changes depending on the functional groups attached to the carbonyl. For example, a benzene ring or double bond in conjugation with the carbonyl will bring the wavenumber down about 30 cm−1.Applications and occurrence
Esters are widespread in nature and are widely used in industry. In nature, fats are, in general, triesters derived from glycerol and fatty acids.[5] Esters are responsible for the aroma of many fruits, including apples, durians, pears, bananas, pineapples, and strawberries.[6] Several billion kilograms of polyesters are produced industrially annually, important products being polyethylene terephthalate, acrylate esters, and cellulose acetate.[7]
Representative triglyceride found in a linseed oil, a triester (triglyceride) derived of linoleic acid, alpha-linolenic acid, and oleic acid.
Preparation
Esterification is the general name for a chemical reaction in which two reactants (typically an alcohol and an acid) form an ester as the reaction product. Esters are common in organic chemistry and biological materials, and often have a characteristic pleasant, fruity odor. This leads to their extensive use in the fragrance and flavor industry. Ester bonds are also found in many polymers.Esterification of carboxylic acids
The classic synthesis is the Fischer esterification, which involves treating a carboxylic acid with an alcohol in the presence of a dehydrating agent:- RCO2H + R'OH
 RCO2R' + H2O
RCO2R' + H2O
- using the alcohol in large excess (i.e., as a solvent)
- using a dehydrating agent: sulfuric acid not only catalyzes the reaction but sequesters water (a reaction product). Other drying agents such as molecular sieves are also effective.
- removal of water by physical means such as distillation as a low-boiling azeotropes with toluene, in conjunction with a Dean-Stark apparatus.
Another method for the dehydration of mixtures of alcohols and carboxylic acids is the Mitsunobu reaction:
- RCO2H + R'OH + P(C6H5)3 + R2N2 → RCO2R' + OP(C6H5)3 + R2N2H2
- RCO2H + CH2N2 → RCO2CH3 + N2
Alcoholysis of acyl chlorides and acid anhydrides
Alcohols react with acyl chlorides and acid anhydrides to give esters:- RCOCl + R'OH → RCO2R' + HCl
- (RCO2)O + R'OH → RCO2R' + RCO2H
Alkylation of carboxylate salts
Although not widely employed for esterifications, salts of carboxylate anions can be alkylating agent with alkyl halides to give esters. In the case that an alkyl chloride is used, an iodide salt can catalyze the reaction (Finkelstein reaction). The carboxylate salt is often generated in situ. In difficult cases, the silver carboxylate may be used, since the silver ion coordinates to the halide aiding its departure and improving the reaction rate. This reaction can suffer from anion availability problems and, therefore, can benefit from the addition of phase transfer catalysts or highly polar aprotic solvents such as DMF.Transesterification
Transesterification, which involves changing one ester into another one, is widely practiced:- RCO2R' + CH3OH → RCO2CH3 + R'OH
-
- (C6H4)(CO2CH3)2 + 2 C2H4(OH)2 → 1/n {(C6H4)(CO2)2(C2H4)}n + 2 CH3OH
Carbonylation
Alkenes undergo "hydroesterification" in the presence of metal carbonyl catalysts. Esters of propionic acid are produced commercially by this method:- C2H4 + ROH + CO → C2H5CO2R
- CH3OH + CO → CH3O2CH
Addition of carboxylic acids to alkenes
In the presence of palladium-based catalysts, ethylene, acetic acid, and oxygen react to give vinyl acetate:- C2H4 + CH3CO2H + 1/2 O2 → C2H3O2CCH3 + H2O
Other methods
- Favorskii rearrangement of α-haloketones in presence of base
- Baeyer-Villiger oxidation of ketones with peroxides
- Pinner reaction of nitriles with an alcohol
- Nucleophilic abstraction of a metal-acyl complex
- Hydrolysis of orthoesters in aqueous acid
- Cellulolysis via esterification [10]
- Ozonolysis of alkenes using a work up in the presence of hydrochloric acid and various alcohols.[11]
- Anodic oxidation of methyl ketones leading to methyl esters.[12]
Reactions
Esters react with nucleophiles at the carbonyl carbon. The carbonyl is weakly electrophilic but is attacked by strong nucleophilies (amines, alkoxides, hydride sources, organolithium compounds, etc.). The C-H bonds adjacent to the carbonyl are weakly acidic but undergo deprotonation with strong bases. This process is the one that usually initiates condensation reactions. The carbonyl oxygen is weakly basic (less so than in amides) but forms adducts.Addition of nucleophiles at carbonyl
Esterification is a reversible reaction. Esters undergo hydrolysis under acid and basic conditions. Under acidic conditions, the reaction is the reverse reaction of the Fischer esterification. Under basic conditions, hydroxide acts as a nucleophile, while an alkoxide is the leaving group. This reaction, saponification, is the basis of soap making.The alkoxide group may also be displaced by stronger nucleophiles such as ammonia or primary or secondary amines to give amides:
-
- RCO2R' + NH2R" → RCONHR" + R'OH
Sources of carbon nucleophiles, e.g., Grignard reagents and organolithium compounds, add readily to the carbonyl.
Reduction
Compared to ketones and aldehydes, esters are relatively resistant to reduction. The introduction of catalytic hydrogenation in the early part of the 20th century was a breakthrough; esters of fatty acids are hydrogenated to fatty alcohols.-
- RCO2R' + 2 H2 → RCH2OH + R'OH
Especially for fine chemical syntheses, lithium aluminium hydride is used to reduce esters to two primary alcohols. The related reagent sodium borohydride is slow in this reaction. DIBAH reduces esters to aldehydes.[13]
Direct reduction to give the corresponding ether is difficult as the intermediate hemiacetal tends to decompose to give an alcohol and an aldehyde (which is rapidly reduced to give a second alcohol). The reaction can be achieved using triethylsilane with a variety of Lewis acids.[14][15]
As for aldehydes, the hydrogen atoms on the carbon adjacent ("α to") the carboxyl group in esters are sufficiently acidic to undergo deprotonation, which in turn leads to a variety of useful reactions. Deprotonation requires relatively strong bases, such as alkoxides. Deprotonation gives a nucleophilic enolate, which can further react, e.g., the Claisen condensation and its intramolecular equivalent, the Dieckmann condensation. This conversion is exploited in the malonic ester synthesis, wherein the diester of malonic acid reacts with an electrophile (e.g., alkyl halide), and is subsequently decarboxylated. Another variation is the Fráter–Seebach alkylation.
Other reactions
- Phenyl esters react to hydroxyarylketones in the Fries rearrangement.
- Specific esters are functionalized with an α-hydroxyl group in the Chan rearrangement.
- Esters with β-hydrogen atoms can be converted to alkenes in ester pyrolysis.
- A direct conversion of esters to nitriles.[16]