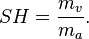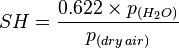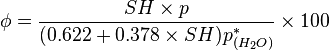From Wikipedia, the free encyclopedia
Humidity is the amount of water vapor in the air. Water vapor is the gaseous state of water and is invisible.[1] Humidity indicates the likelihood of precipitation, dew, or fog. Higher humidity reduces the effectiveness of sweating in cooling the body by reducing the rate of evaporation of moisture from the skin. This effect is calculated in a heat index table or humidex, used during summer weather.
There are three main measurements of humidity: absolute, relative and specific. Absolute humidity is the water content of air.[2] Relative humidity, expressed as a percent, measures the current absolute humidity relative to the maximum for that temperature. Specific humidity is a ratio of the water vapor content of the mixture to the total air content on a mass basis.
Types
Absolute humidity
Absolute humidity is the total amount of water vapor present in a given volume of air. It does not take temperature into consideration. Absolute humidity in the atmosphere ranges from near zero to roughly 30 grams per cubic meter when the air is saturated at 30 °C.[4]Absolute humidity is the mass of the water vapor (
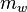 ), divided by the volume of the air and water vapor mixture (
), divided by the volume of the air and water vapor mixture ( ), which can be expressed as:
), which can be expressed as:The field concerned with the study of physical and thermodynamic properties of gas–vapor mixtures is named psychrometrics.
Relative humidity
The relative humidity of an air-water mixture is defined as the ratio of the partial pressure of water vapor (H2O)
of an air-water mixture is defined as the ratio of the partial pressure of water vapor (H2O)  in the mixture to the saturated vapor pressure of water
in the mixture to the saturated vapor pressure of water  at a given temperature. Thus the relative humidity of air is a function of both water content and temperature.
at a given temperature. Thus the relative humidity of air is a function of both water content and temperature.Relative humidity is normally expressed as a percentage and is calculated by using the following equation:[5]
Specific humidity
Specific humidity (or moisture content) is the ratio of water vapor mass ( ) to the air parcel's total (i.e., including dry) mass (
) to the air parcel's total (i.e., including dry) mass ( ) and is sometimes referred to as the humidity ratio.[8] Specific humidity is approximately equal to the "mixing ratio", which is defined as the ratio of the mass of water vapor in an air parcel to the mass of dry air for the same parcel.[8]
) and is sometimes referred to as the humidity ratio.[8] Specific humidity is approximately equal to the "mixing ratio", which is defined as the ratio of the mass of water vapor in an air parcel to the mass of dry air for the same parcel.[8]Specific Humidity is defined as:
Measurement
There are various devices used to measure and regulate humidity. A device used to measure humidity is called a psychrometer or hygrometer. A humidistat is a humidity-triggered switch, often used to control a dehumidifier.
Humidity is also measured on a global scale using remotely placed satellites. These satellites are able to detect the concentration of water in the troposphere at altitudes between 4 and 12 kilometers. Satellites that can measure water vapor have sensors that are sensitive to infrared radiation. Water vapor specifically absorbs and re-radiates radiation in this spectral band. Satellite water vapor imagery plays an important role in monitoring climate conditions (like the formation of thunderstorms) and in the development of future weather forecasts.
Climate
While humidity itself is a climate variable, it also interacts strongly with other climate variables. The humidity is affected by winds and by rainfall. At the same time, humidity affects the energy budget and thereby influences temperatures in two major ways. First, water vapor in the atmosphere contains "latent" energy. During transpiration or evaporation, this latent heat is removed from surface liquid, cooling the earth's surface. This is the biggest non-radiative cooling effect at the surface. It compensates for roughly 70% of the average net radiative warming at the surface.Second, water vapor is the most abundant of all greenhouse gases. Water vapor, like a green lens that allows green light to pass through it but absorbs red light, is a "selective absorber". Along with other greenhouse gases, water vapor is transparent to most solar energy, as you can literally see. But it absorbs the infrared energy emitted (radiated) upward by the earth's surface, which is the reason that humid areas experience very little nocturnal cooling but dry desert regions cool considerably at night. This selective absorption causes the greenhouse effect. It raises the surface temperature substantially above its theoretical radiative equilibrium temperature with the sun, and water vapor is the cause of more of this warming than any other greenhouse gas.
Unlike most other greenhouse gases, however, water is not merely below its boiling point in all regions of the Earth, but below its freezing point at many altitudes. As a condensible greenhouse gas, it precipitates, with a much lower scale height and shorter atmospheric lifetime- weeks instead of decades. Without other greenhouse gases, Earth's blackbody temperature, below the freezing point of water, would cause water vapor to be removed from the atmosphere.[10][11][12] Water vapor is thus a "slave" to the non-condensible greenhouse gases.[13][14][15]
The most humid cities on earth are generally located closer to the equator, near coastal regions. Cities in South and Southeast Asia are among the most humid. Kuala Lumpur and Singapore have very high humidity all year round because of their proximity to water bodies and the equator and often overcast weather. Some places experience extreme humidity during their rainy seasons combined with warmth giving the feel of a lukewarm sauna, such as Kolkata, Chennai and Cochin in India, and Lahore in Pakistan. Sukkur city located on the Indus River in Pakistan has some of the highest and most uncomfortable dew point in the country frequently exceeding 30 °C (86 °F) in the Monsoon season.[16] High temperatures couple up with bizarre dew point to create heat index in excess of 65 °C (149 °F). The cities of Manila in the Philippines, Mogadishu in Somalia and Bangkok in Thailand. Darwin, Australia experiences an extremely humid wet season from December to April. Shanghai and Hong Kong in China also have an extreme humid period in their summer months. During the South-west and North-east Monsoon seasons (respectively, late May to September and November to March), expect heavy rains and a relatively high humidity post-rainfall. Outside the monsoon seasons, humidity is high (in comparison to countries North of the Equator), but completely sunny days abound. In cooler places such as Northern Tasmania, Australia, high humidity is experienced all year due to the ocean between mainland Australia and Tasmania. In the summer the hot dry air is absorbed by this ocean and the temperature rarely climbs above 35 °C (95 °F).
In the United States the most humid cities, strictly in terms of relative humidity, are Forks and Olympia, Washington.[17] This fact may come as a surprise to many, as the climate in this region rarely exhibits the discomfort usually associated with high humidity. This is because high dew points play a more significant role than relative humidity in discomfort, and so the air in these western cities usually does not feel "humid" as a result. In general, dew points are much lower in the Western U.S. than those in the Eastern U.S.
The highest dew points in the US are found in coastal Florida and Texas. When comparing Key West and Houston, two of the most humid cities from those states, coastal Florida seems to have the higher dew points on average. However, Houston lacks the coastal breeze present in Key West, and, as a much larger city, it suffers from the urban heat island effect.[18] A dew point of 88 °F (31 °C) was recorded in Moorhead Minnesota on July 19, 2011, with a heat index of 133.5, although dew points over 80 °F (27 °C) are rare there.[19] The US city with the lowest annual humidity is Las Vegas, Nevada, averaging 39% for a high and 21% as a low.[20]
Air density and volume
Humidity depends on water vaporization and condensation, which, in turn, mainly depends on temperature. Therefore, when applying more pressure to a gas saturated with water, all components will initially decrease in volume approximately according to the ideal gas law. However, some of the water will condense until returning to almost the same humidity as before, giving the resulting total volume deviating from what the ideal gas law predicted. Conversely, decreasing temperature would also make some water condense, again making the final volume deviate from predicted by the ideal gas law. Therefore, gas volume may alternatively be expressed as the dry volume, excluding the humidity content. This fraction more accurately follows the ideal gas law. On the contrary the saturated volume is the volume a gas mixture would have if humidity was added to it until saturation (or 100% relative humidity).Humid air is less dense than dry air because a molecule of water (M ≈ 18 u) is less massive than either a molecule of nitrogen (M ≈ 28) or a molecule of oxygen (M ≈ 32). About 78% of the molecules in dry air are nitrogen (N2). Another 21% of the molecules in dry air are oxygen (O2). The final 1% of dry air is a mixture of other gases.
For any gas, at a given temperature and pressure, the number of molecules present in a particular volume is constant – see ideal gas law. So when water molecules (vapor) are introduced into that volume of dry air, the number of air molecules in the volume must decrease by the same number, if the temperature and pressure remain constant. (The addition of water molecules, or any other molecules, to a gas, without removal of an equal number of other molecules, will necessarily require a change in temperature, pressure, or total volume; that is, a change in at least one of these three parameters. If temperature and pressure remain constant, the volume increases, and the dry air molecules that were displaced will initially move out into the additional volume, after which the mixture will eventually become uniform through diffusion.) Hence the mass per unit volume of the gas—its density—decreases. Isaac Newton discovered this phenomenon and wrote about it in his book Opticks.[21]
Effects
Animals and plants
Humidity is one of the fundamental abiotic factors that defines any habitat, and is a determinant of which animals and plants can thrive in a given environment.[22]The human body dissipates heat through perspiration and its evaporation. Heat convection to the surrounding air, and thermal radiation are the primary modes of heat transport from the body. Under conditions of high humidity, the rate of evaporation of sweat from the skin decreases. Also, if the atmosphere is as warm as or warmer than the skin during times of high humidity, blood brought to the body surface cannot dissipate heat by conduction to the air, and a condition called hyperpyrexia results. With so much blood going to the external surface of the body, relatively less goes to the active muscles, the brain, and other internal organs. Physical strength declines, and fatigue occurs sooner than it would otherwise. Alertness and mental capacity also may be affected, resulting in heat stroke or hyperthermia.
Human comfort
Humans are sensitive to humid air because the human body uses evaporative cooling as the primary mechanism to regulate temperature. Under humid conditions, the rate at which perspiration evaporates on the skin is lower than it would be under arid conditions. Because humans perceive the rate of heat transfer from the body rather than temperature itself, we feel warmer when the relative humidity is high than when it is low.Some people experience difficulty breathing in high humidity environments. Some cases may possibly be related to respiratory conditions such as asthma, while others may be the product of anxiety. Sufferers will often hyperventilate in response, causing sensations of numbness, faintness, and loss of concentration, among others.[citation needed]
Air conditioning reduces discomfort in the summer not only by reducing temperature, but also by reducing humidity. In winter, heating cold outdoor air can decrease relative humidity levels indoor to below 30%, leading to discomfort such as dry skin and excessive thirst.
Electronics
Many electronic devices have humidity specifications, for example, 5% to 95%. At the top end of the range, moisture may increase the conductivity of permeable insulators leading to malfunction. Too low humidity may make materials brittle. A particular danger to electronic items, regardless of the stated operating humidity range, is condensation. When an electronic item is moved from a cold place (e.g. garage, car, shed, an air conditioned space in the tropics) to a warm humid place (house, outside tropics), condensation may coat circuit boards and other insulators, leading to short circuit inside the equipment. Such short circuits may cause substantial permanent damage if the equipment is powered on before the condensation has evaporated. A similar condensation effect can often be observed when a person wearing glasses comes in from the cold (i.e. the glasses become foggy).[23] It is advisable to allow electronic equipment to acclimatise for several hours, after being brought in from the cold, before powering on. Some electronic devices can detect such a change and indicate, when plugged in and usually with a small droplet symbol, that they cannot be used until the risk from condensation has passed. In situations where time is critical, increasing air flow through the device's internals, such as removing the side panel from a PC case and directing a fan to blow into the case, will reduce significantly the time needed to acclimatise to the new environment.In contrast, a very low humidity level favors the build-up of static electricity, which may result in spontaneous shutdown of computers when discharges occur. Apart from spurious erratic function, electrostatic discharges can cause dielectric breakdown in solid state devices, resulting in irreversible damage. Data centers often monitor relative humidity levels for these reasons.