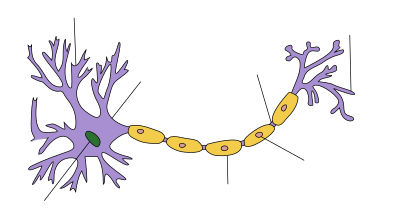
| Myelin sheath |
|---|
Myelin is a lipid-rich substance that surrounds the axon of some nerve cells, forming an electrically insulating layer. It is essential for the proper functioning of the nervous system. It is produced by specialized glial cells through extension of their cellular processes.
The production of the myelin sheath is called myelination or myelinogenesis. In humans, myelination begins early in the 3rd trimester,[1] although little myelin exists in the brain at the time of birth. During infancy, myelination occurs quickly, leading to a child's fast development, including crawling and walking in the first year. Myelination continues through the adolescent stage of life.
Schwann cells supply the myelin for the peripheral nervous system, whereas oligodendrocytes, specifically of the interfascicular type, myelinate the axons of the central nervous system. Myelin is considered a defining characteristic of the (gnathostome) vertebrates, but myelin-like sheaths have also been seen in some invertebrates,[2][3] although they are quite different from vertebrate myelin at molecular level. Myelin was discovered in 1854 by Rudolf Virchow.[4]
Composition
Transmission electron micrograph of a cross-section of a myelinated axon, generated at the Electron Microscopy Facility at Trinity College, Hartford CT
Myelin comprises different cell types and varies in chemical composition and configuration but performs the same insulating function. Myelinated axons are white; hence, the "white matter" of the brain. Myelin insulates axons from electrically charged atoms and molecules. These charged particles (ions) are found in the fluid surrounding the entire nervous system. Under a microscope, myelin looks like strings of sausages.
Cholesterol is an essential component of myelin,[5] which comprises about 40% water; the dry mass comprises between 60% and 75% lipids and between 15% and 25% proteins. Myelin basic protein (MBP) constitutes ~23% of myelin protein,[6] myelin oligodendrocyte glycoprotein, and proteolipid protein (PLP, which makes up ~50% of myelin protein[7]). The primary lipid of myelin is a glycolipid called galactocerebroside. The intertwining hydrocarbon chains of sphingomyelin strengthen the myelin sheath. In brain, the myelin sheath covers the fibers of the corpus callosum, which constitute the inner part of the cerebral hemisphere.
Function
Action potential propagation in myelinated neurons is faster than in unmyelinated neurons because of Saltatory conduction.
Cross section of a myelinated axon
1. Axon
2. Nucleus of Schwann Cell
3. Schwann Cell
4. Myelin Sheath
5. Neurilemma
1. Axon
2. Nucleus of Schwann Cell
3. Schwann Cell
4. Myelin Sheath
5. Neurilemma
The main purpose of a myelin sheath is to increase the speed at which impulses propagate along the myelinated fiber. Along unmyelinated fibers, impulses continuously move as waves, but, in myelinated fibers, they "hop" or propagate by saltatory conduction. Myelin decreases capacitance and increases electrical resistance across the cell membrane (the axolemma). Thus, myelination prevents the electric current from leaving the axon. It has been suggested that myelin permits larger body size by maintaining agile communication between distant body parts.[2]
Myelinated fibers lack voltage-gated ion channels (approximately 25 μm−2) along the myelinated internodes, exposing them only at the nodes of Ranvier. Here, they are found far more abundantly (between 2,000 and 12,000 μm−2).[8] Myelinated fibers succeed in reducing sodium leakage into the extracellular fluid (ECF), maintaining a strong separation of charge between the intracellular fluid (ICF) and the ECF. This increases sodium's ability to travel along the axon more freely. However, the sodium diffuses along the axolemma rapidly, but is decremental by nature. The sodium cannot trigger the opening of the voltage-gated sodium channels as it becomes weaker. The nodes of Ranvier, being exposed to the ECF every 1 mm or so, contain large amounts of voltage-gated sodium channels, and allow enough sodium into the axon to regenerate the action potential.[9] Each time the action potential reaches a node of Ranvier, it is restored to its original action potential (+35 mV).[8]
When a peripheral fiber is severed, the myelin sheath provides a track along which regrowth can occur. However, the myelin layer does not ensure a perfect regeneration of the nerve fiber. Some regenerated nerve fibers do not find the correct muscle fibers, and some damaged motor neurons of the peripheral nervous system die without regrowth. Damage to the myelin sheath and nerve fiber is often associated with increased functional insufficiency.
Unmyelinated fibers and myelinated axons of the mammalian central nervous system do not regenerate.
Some studies have revealed that optic nerve fibers can be regenerated in postnatal rats. This regeneration depends upon two conditions: axonal die-back has to be prevented with appropriate neurotrophic factors, and neurite growth inhibitory components have to be inactivated. These studies may lead to further understanding of nerve fiber regeneration in the central nervous system.[citation needed]
Disorders
Demyelination
Demyelination is the loss of the myelin sheath insulating the nerves, and is the hallmark of some neurodegenerative autoimmune diseases, including multiple sclerosis, acute disseminated encephalomyelitis, neuromyelitis optica, transverse myelitis, chronic inflammatory demyelinating polyneuropathy, Guillain–Barré syndrome, central pontine myelinosis, inherited demyelinating diseases such as leukodystrophy, and Charcot-Marie-Tooth disease. Sufferers of pernicious anaemia can also suffer nerve damage if the condition is not diagnosed quickly. Subacute combined degeneration of spinal cord secondary to pernicious anaemia can lead to slight peripheral nerve damage to severe damage to the central nervous system, affecting speech, balance, and cognitive awareness. When myelin degrades, conduction of signals along the nerve can be impaired or lost, and the nerve eventually withers.[clarification needed] A more serious case of myelin deterioration is called Canavan disease.The immune system may play a role in demyelination associated with such diseases, including inflammation causing demyelination by overproduction of cytokines via upregulation of tumor necrosis factor[10] or interferon.
Symptoms
Demyelination results in diverse symptoms determined by the functions of the affected neurons. It disrupts signals between the brain and other parts of the body; symptoms differ from patient to patient, and have different presentations upon clinical observation and in laboratory studies.Typical symptoms include:
- blurriness in the central visual field that affects only one eye, may be accompanied by pain upon eye movement
- double vision
- loss of vision/hearing
- odd sensation in legs, arms, chest, or face, such as tingling or numbness (neuropathy)
- weakness of arms or legs
- cognitive disruption, including speech impairment and memory loss
- heat sensitivity (symptoms worsen or reappear upon exposure to heat, such as a hot shower)
- loss of dexterity
- difficulty coordinating movement or balance disorder
- difficulty controlling bowel movements or urination
- fatigue
- tinnitus[11]