Structure of a eukaryotic gene
In computational biology, gene prediction or gene finding refers to the process of identifying the regions of genomic DNA that encode genes. This includes protein-coding genes as well as RNA genes, but may also include prediction of other functional elements such as regulatory regions. Gene finding is one of the first and most important steps in understanding the genome of a species once it has been sequenced.
In its earliest days, "gene finding" was based on painstaking
experimentation on living cells and organisms. Statistical analysis of
the rates of homologous recombination of several different genes could determine their order on a certain chromosome, and information from many such experiments could be combined to create a genetic map
specifying the rough location of known genes relative to each other.
Today, with comprehensive genome sequence and powerful computational
resources at the disposal of the research community, gene finding has
been redefined as a largely computational problem.
Determining that a sequence is functional should be distinguished from determining the function
of the gene or its product. Predicting the function of a gene and
confirming that the gene prediction is accurate still demands in vivo experimentation through gene knockout and other assays, although frontiers of bioinformatics research are making it increasingly possible to predict the function of a gene based on its sequence alone.
Gene prediction is one of the key steps in genome annotation, following sequence assembly, the filtering of non-coding regions and repeat masking.
Gene prediction is closely related to the so-called 'target search problem' investigating how DNA-binding proteins (transcription factors) locate specific binding sites within the genome. Many aspects of structural gene prediction are based on current understanding of underlying biochemical processes in the cell such as gene transcription, translation, protein–protein interactions and regulation processes, which are subject of active research in the various omics fields such as transcriptomics, proteomics, metabolomics, and more generally structural and functional genomics.
Empirical methods
In
empirical (similarity, homology or evidence-based) gene finding
systems, the target genome is searched for sequences that are similar to
extrinsic evidence in the form of the known expressed sequence tags, messenger RNA (mRNA), protein
products, and homologous or orthologous sequences. Given an mRNA
sequence, it is trivial to derive a unique genomic DNA sequence from
which it had to have been transcribed. Given a protein sequence, a family of possible coding DNA sequences can be derived by reverse translation of the genetic code.
Once candidate DNA sequences have been determined, it is a relatively
straightforward algorithmic problem to efficiently search a target
genome for matches, complete or partial, and exact or inexact. Given a
sequence, local alignment algorithms such as BLAST, FASTA and Smith-Waterman
look for regions of similarity between the target sequence and possible
candidate matches. Matches can be complete or partial, and exact or
inexact. The success of this approach is limited by the contents and
accuracy of the sequence database.
A high degree of similarity to a known messenger RNA or protein
product is strong evidence that a region of a target genome is a
protein-coding gene. However, to apply this approach systemically
requires extensive sequencing of mRNA and protein products. Not only is
this expensive, but in complex organisms, only a subset of all genes in
the organism's genome are expressed at any given time, meaning that
extrinsic evidence for many genes is not readily accessible in any
single cell culture. Thus, to collect extrinsic evidence for most or all
of the genes in a complex organism requires the study of many hundreds
or thousands of cell types,
which presents further difficulties. For example, some human genes may
be expressed only during development as an embryo or fetus, which might
be difficult to study for ethical reasons.
Despite these difficulties, extensive transcript and protein
sequence databases have been generated for human as well as other
important model organisms in biology, such as mice and yeast. For
example, the RefSeq database contains transcript and protein sequence from many different species, and the Ensembl
system comprehensively maps this evidence to human and several other
genomes. It is, however, likely that these databases are both incomplete
and contain small but significant amounts of erroneous data.
New high-throughput transcriptome sequencing technologies such as RNA-Seq and ChIP-sequencing
open opportunities for incorporating additional extrinsic evidence into
gene prediction and validation, and allow structurally rich and more
accurate alternative to previous methods of measuring gene expression such as expressed sequence tag or DNA microarray.
Major challenges involved in gene prediction involve dealing with
sequencing errors in raw DNA data, dependence on the quality of the sequence assembly, handling short reads, frameshift mutations, overlapping genes and incomplete genes.
In prokaryotes it's essential to consider horizontal gene transfer
when searching for gene sequence homology. An additional important
factor underused in current gene detection tools is existence of gene
clusters—operons
in both prokaryotes and eukaryotes. Most popular gene detectors treat
each gene in isolation, independent of others, which is not biologically
accurate.
Ab initio methods
Ab
Initio gene prediction is an intrinsic method based on gene content and
signal detection. Because of the inherent expense and difficulty in
obtaining extrinsic evidence for many genes, it is also necessary to
resort to ab initio gene finding, in which the genomic DNA sequence
alone is systematically searched for certain tell-tale signs of
protein-coding genes. These signs can be broadly categorized as either signals, specific sequences that indicate the presence of a gene nearby, or content, statistical properties of the protein-coding sequence itself. Ab initio gene finding might be more accurately characterized as gene prediction, since extrinsic evidence is generally required to conclusively establish that a putative gene is functional.
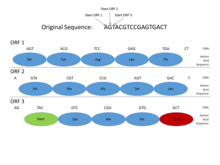
This
picture shows how Open Reading Frames (ORFs) can be used for gene
prediction. Gene prediction is the process of determining where a coding
gene might be in a genomic sequence. Functional proteins must begin
with a Start codon (where DNA transcription begins), and end with a Stop
codon (where transcription ends). By looking at where those codons
might fall in a DNA sequence, one can see where a functional protein
might be located. This is important in gene prediction because it can
reveal where coding genes are in an entire genomic sequence. In this
example, a functional protein can be discovered using ORF3 because it
begins with a Start codon, has multiple amino acids, and then ends with a
Stop codon, all within the same reading frame.
In the genomes of prokaryotes, genes have specific and relatively well-understood promoter sequences (signals), such as the Pribnow box and transcription factor binding sites, which are easy to systematically identify. Also, the sequence coding for a protein occurs as one contiguous open reading frame (ORF), which is typically many hundred or thousands of base pairs long. The statistics of stop codons
are such that even finding an open reading frame of this length is a
fairly informative sign. (Since 3 of the 64 possible codons in the
genetic code are stop codons, one would expect a stop codon
approximately every 20–25 codons, or 60–75 base pairs, in a random sequence.) Furthermore, protein-coding DNA has certain periodicities
and other statistical properties that are easy to detect in sequence of
this length. These characteristics make prokaryotic gene finding
relatively straightforward, and well-designed systems are able to
achieve high levels of accuracy.
Ab initio gene finding in eukaryotes,
especially complex organisms like humans, is considerably more
challenging for several reasons. First, the promoter and other
regulatory signals in these genomes are more complex and less
well-understood than in prokaryotes, making them more difficult to
reliably recognize. Two classic examples of signals identified by
eukaryotic gene finders are CpG islands and binding sites for a poly(A) tail.
Second, splicing
mechanisms employed by eukaryotic cells mean that a particular
protein-coding sequence in the genome is divided into several parts (exons), separated by non-coding sequences (introns).
(Splice sites are themselves another signal that eukaryotic gene
finders are often designed to identify.) A typical protein-coding gene
in humans might be divided into a dozen exons, each less than two
hundred base pairs in length, and some as short as twenty to thirty. It
is therefore much more difficult to detect periodicities and other known
content properties of protein-coding DNA in eukaryotes.
Advanced gene finders for both prokaryotic and eukaryotic genomes typically use complex probabilistic models, such as hidden Markov models (HMMs) to combine information from a variety of different signal and content measurements. The GLIMMER system is a widely used and highly accurate gene finder for prokaryotes. GeneMark is another popular approach. Eukaryotic ab initio gene finders, by comparison, have achieved only limited success; notable examples are the GENSCAN and geneid
programs. The SNAP gene finder is HMM-based like Genscan, and attempts
to be more adaptable to different organisms, addressing problems related
to using a gene finder on a genome sequence that it was not trained
against. A few recent approaches like mSplicer, CONTRAST, or mGene also use machine learning techniques like support vector machines for successful gene prediction. They build a discriminative model using hidden Markov support vector machines or conditional random fields to learn an accurate gene prediction scoring function.
Ab Initio methods have been benchmarked, with some approaching 100% sensitivity, however as the sensitivity increases, accuracy suffers as a result of increased false positives.
Other signals
Among the derived signals used for prediction are statistics resulting from the sub-sequence statistics like k-mer statistics, Isochore (genetics) or Compositional domain GC composition/uniformity/entropy, sequence and frame length, Intron/Exon/Donor/Acceptor/Promoter and Ribosomal binding site vocabulary, Fractal dimension, Fourier transform of a pseudo-number-coded DNA, Z-curve parameters and certain run features.
It has been suggested that signals other than those directly
detectable in sequences may improve gene prediction. For example, the
role of secondary structure in the identification of regulatory motifs has been reported. In addition, it has been suggested that RNA secondary structure prediction helps splice site prediction.
Neural networks
Artificial neural networks are computational models that excel at machine learning and pattern recognition. Neural networks must be trained
with example data before being able to generalise for experimental
data, and tested against benchmark data. Neural networks are able to
come up with approximate solutions to problems that are hard to solve
by algorithms, provided there is sufficient training data. When
applied to gene prediction, neural networks can be used alongside other ab initio methods to predict or identify biological features such as splice sites. One approach
involves using a sliding window, which traverses the sequence data in
an overlapping manner. The output at each position is a score based on
whether the network thinks the window contains a donor splice site or an
acceptor splice site. Larger windows offer more accuracy but also
require more computational power. A neural network is an example of a
signal sensor as its goal is to identify a functional site in the
genome.
Combined approaches
Programs such as Maker combine extrinsic and ab initio approaches by mapping protein and EST data to the genome to validate ab initio predictions. Augustus,
which may be used as part of the Maker pipeline, can also incorporate
hints in the form of EST alignments or protein profiles to increase the
accuracy of the gene prediction.
Comparative genomics approaches
As the entire genomes of many different species are sequenced, a promising direction in current research on gene finding is a comparative genomics approach.
This is based on the principle that the forces of natural selection
cause genes and other functional elements to undergo mutation at a
slower rate than the rest of the genome, since mutations in functional
elements are more likely to negatively impact the organism than
mutations elsewhere. Genes can thus be detected by comparing the
genomes of related species to detect this evolutionary pressure for
conservation. This approach was first applied to the mouse and human
genomes, using programs such as SLAM, SGP and TWINSCAN/N-SCAN and
CONTRAST.
Multiple informants
TWINSCAN
examined only human-mouse synteny to look for orthologous genes.
Programs such as N-SCAN and CONTRAST allowed the incorporation of
alignments from multiple organisms, or in the case of N-SCAN, a single
alternate organism from the target. The use of multiple informants can
lead to significant improvements in accuracy.
CONTRAST is composed of two elements. The first is a smaller
classifier, identifying donor splice sites and acceptor splice sites as
well as start and stop codons. The second element involves constructing a
full model using machine learning. Breaking the problem into two means
that smaller targeted data sets can be used to train the classifiers,
and that classifier can operate independently and be trained with
smaller windows. The full model can use the independent classifier, and
not have to waste computational time or model complexity re-classifying
intron-exon boundaries. The paper in which CONTRAST is introduced
proposes that their method (and those of TWINSCAN, etc.) be classified
as de novo gene assembly, using alternate genomes, and identifying it as distinct from ab initio, which uses a target 'informant' genomes.
Comparative gene finding can also be used to project high quality
annotations from one genome to another. Notable examples include
Projector, GeneWise, GeneMapper and GeMoMa. Such techniques now play a
central role in the annotation of all genomes.
Pseudogene prediction
Pseudogenes are close relatives of genes, sharing very high sequence homology, but being unable to code for the same protein product. Whilst once relegated as byproducts of gene sequencing, increasingly, as regulatory roles are being uncovered, they are becoming predictive targets in their own right.
Pseudogene prediction utilizes existing sequence similarity and ab
initio methods, whilst adding additional filtering and methods of
identifying pseudogene characteristics.
Sequence similarity methods can be customized for pseudogene
prediction using additional filtering to find candidate pseudogenes.
This could use disablement detection, which looks for nonsense or
frameshift mutations that would truncate or collapse an otherwise
functional coding sequence. Additionally, translating DNA into proteins sequences can be more effective than just straight DNA homology.
Content sensors can be filtered according to the differences in
statistical properties between pseudogenes and genes, such as a reduced
count of CpG islands in pseudogenes, or the differences in G-C content
between pseudogenes and their neighbours. Signal sensors also can be
honed to pseudogenes, looking for the absence of introns or polyadenine
tails.
Metagenomic gene prediction
Metagenomics
is the study of genetic material recovered from the environment,
resulting in sequence information from a pool of organisms. Predicting
genes is useful for comparative metagenomics.
Metagenomics tools also fall into the basic categories of using
either sequence similarity approaches (MEGAN4) and ab initio techniques
(GLIMMER-MG).
Glimmer-MG is an extension to GLIMMER
that relies mostly on an ab initio approach for gene finding and by
using training sets from related organisms. The prediction strategy is
augmented by classification and clustering gene data sets prior to
applying ab initio gene prediction methods. The data is clustered by
species. This classification method leverages techniques from
metagenomic phylogenetic classification. An example of software for this
purpose is, Phymm, which uses interpolated markov models—and PhymmBL,
which integrates BLAST into the classification routines.
MEGAN4
uses a sequence similarity approach, using local alignment against
databases of known sequences, but also attempts to classify using
additional information on functional roles, biological pathways and
enzymes. As in single organism gene prediction, sequence similarity
approaches are limited by the size of the database.
FragGeneScan and MetaGeneAnnotator are popular gene prediction programs based on Hidden Markov model. These predictors account for sequencing errors, partial genes and work for short reads.
Another fast and accurate tool for gene prediction in metagenomes is MetaGeneMark. This tool is used by the DOE Joint Genome Institute to annotate IMG/M, the largest metagenome collection to date.