Hybrid solar cells combine advantages of both organic and inorganic semiconductors. Hybrid photovoltaics have organic materials that consist of conjugated polymers that absorb light as the donor and transport holes. Inorganic materials in hybrid cells are used as the acceptor and electron transporter in the structure. The hybrid photovoltaic devices have a potential for not only low-cost by roll-to-roll processing but also for scalable solar power conversion.
Theory
Solar cells are devices that convert sunlight into electricity by the photovoltaic effect. Electrons in a solar cell absorb photon energy in sunlight which excites them to the conduction band from the valence band. This generates a hole-electron pair, which is separated by a potential barrier (such as a p-n junction), and induces a current. Organic solar cells
use organic materials in their active layers. Molecular, polymer, and
hybrid organic photovoltaics are the main kinds of organic photovoltaic
devices currently studied.
Hybrid solar cell
Figure 1. Energy diagram of the donor and acceptor. The conduction band of the acceptor is lower than the LUMO of the polymer, allowing for transfer of the electron.
In hybrid solar cells, an organic material is mixed with a high electron transport material to form the photoactive layer. The two materials are assembled together in a heterojunction-type photoactive layer, which can have a greater power conversion efficiency than a single material. One of the materials acts as the photon absorber and exciton
donor. The other material facilitates exciton dissociation at the
junction. Charge is transferred and then separated after an exciton
created in the donor is delocalized on a donor-acceptor complex.
The acceptor material needs a suitable energy offset to the
binding energy of the exciton to the absorber. Charge transfer is
favorable if the following condition is satisfied:
where superscripts A and D refer to the acceptor and donor respectively, EA
is the electron affinity, and U the coulombic binding energy of the
exciton on the donor. An energy diagram of the interface is shown in
figure 1. In commonly used photovoltaic polymers such as MEH-PPV, the
exciton binding energy ranges from 0.3 eV to 1.4 eV.
The energy required to separate the exciton is provided by the energy offset between the LUMOs or conduction bands of the donor and acceptor. After dissociation, the carriers are transported to the respective electrodes through a percolation network.
The average distance an exciton can diffuse through a material
before annihilation by recombination is the exciton diffusion length.
This is short in polymers, on the order of 5–10 nanometers. The time scale for radiative and non-radiative decay is from 1 picosecond to 1 nanosecond. Excitons generated within this length close to an acceptor would contribute to the photocurrent.
Figure
2. Two different structures of heterojunctions, a) phase separated
bi-layer and b) bulk heterojunction. The bulk heterojunction allows for
more interfacial contact between the two phases, which is beneficial for
the nanoparticle-polymer compound as it provides more surface area for charge transfer.
To deal with the problem of the short exciton diffusion length, a
bulk heterojunction structure is used rather than a phase-separated
bilayer. Dispersing the particles throughout the polymer matrix creates a
larger interfacial area for charge transfer to occur. Figure 2 displays the difference between a bilayer and a bulk heterojunction.
Types of interfaces and structures
Controlling
the interface of inorganic-organic hybrid solar cells can increase the
efficiency of the cells. This increased efficiency can be achieved by
increasing the interfacial surface area between the organic and the
inorganic to facilitate charge separation and by controlling the
nanoscale lengths and periodicity of each structure so that charges are
allowed to separate and move toward the appropriate electrode without
recombining. The three main nanoscale structures used are mesoporous
inorganic films infused with electron-donating organic, alternatining
inorganic-organic lamellar structures, and nanowire structures.
Mesoporous films
Mesoporous films have been used for a relatively high-efficiency hybrid solar cell. The structure of mesoporous thin film solar cells
usually includes a porous inorganic that is saturated with organic
surfactant. The organic absorbs light, and transfers electrons to the
inorganic semiconductor (usually a transparent conducting oxide), which
then transfers the electron to the electrode. Problems with these cells
include their random ordering and the difficulty of controlling their
nanoscale structure to promote charge conduction.
Ordered lamellar films
Recently,
the use of alternating layers of organic and inorganic compounds has
been controlled through electrodeposition-based self-assembly.
This is of particular interest because it has been shown that the
lamellar structure and periodicity of the alternating organic-inorganic
layers can be controlled through solution chemistry. To produce this
type of cell with practical efficiencies, larger organic surfactants
that absorb more of the visible spectrum must be deposited between the
layers of electron-accepting inorganic.
Films of ordered nanostructures
Researchers
have been able to grow nanostructure-based solar cells that use ordered
nanostructures like nanowires or nanotubes of inorganic surrounding by
electron-donating organics utilizing self-organization processes.
Ordered nanostructures offer the advantage of directed charge transport
and controlled phase separation between donor and acceptor materials.
The nanowire-based morphology offers reduced internal reflection,
facile strain relaxation and increased defect tolerance. The ability to
make single-crystalline nanowires on low-cost substrates such as
aluminum foil and to relax strain in subsequent layers removes two more
major cost hurdles associated with high-efficiency cells. There have
been rapid increases in efficiencies of nanowire-based solar cells and
they seem to be one of the most promising nanoscale solar hybrid
technologies.
Fundamental challenge factors
Hybrid cell efficiency must be increased to start large-scale manufacturing. Three factors affect efficiency.
First, the bandgap should be reduced to absorb red photons, which
contain a significant fraction of the energy in the solar spectrum.
Current organic photovoltaics have shown 70% of quantum efficiency for
blue photons. Second, contact resistance between each layer in the
device should be minimized to offer higher fill factor and power
conversion efficiency. Third, charge-carrier mobility should be
increased to allow the photovoltaics to have thicker active layers while
minimizing carrier recombination and keeping the series resistance of
the device low.
Types of hybrid solar cells
Polymer–nanoparticle composite
Nanoparticles
are a class of semiconductor materials whose size in at least one
dimension ranges from 1 to 100 nanometers, on the order of exciton
wavelengths. This size control creates quantum confinement and allows
for the tuning of optoelectronic properties, such as band gap and
electron affinity. Nanoparticles also have a large surface area to
volume ratio, which presents more area for charge transfer to occur.
The photoactive layer can be created by mixing nanoparticles into
a polymer matrix. Solar devices based on polymer-nanoparticle
composites most resemble polymer solar cells.
In this case, the nanoparticles take the place of the fullerene based
acceptors used in fully organic polymer solar cells. Hybrid solar cells
based upon nanoparticles are an area of research interest because
nanoparticles have several properties that could make them preferable to
fullerenes, such as:
- Fullerenes are synthesized by a combination of a high temperature arc method and continuous gas-phase synthesis, which makes their production difficult and energy intensive. The colloidal synthesis of nanoparticles by contrast is a low temperature process.
- PCBM (a common fullerene acceptor) diffuses during long timespans or when exposed to heat, which can alter the morphology and lower the efficiency of a polymer solar cell. Limited testing of nanoparticle solar cells indicates they may be more stable over time.
- Nanoparticles are more absorbent than fullerenes, meaning more light can be theoretically absorbed in a thinner device.
- Nanoparticle size can affect absorption. This combined with the fact that there are many possible semiconducting nanoparticles allows for highly customizable bandgaps that can be easily tuned to certain frequencies, which would be advantageous in tandem solar cells.
- Nanoparticles with size near their Bohr radius can generate two excitons when struck by a sufficiently energetic photon.
Structure and processing
Figure
3. Four different structures of nanoparticles, which have at least 1
dimension in the 1–100 nm range, retaining quantum confinement. Left is a
nanocrystal, next to it is nanorod, third is tetrapod, and right is
hyperbranched.
For polymers used in this device, hole mobilities are greater than
electron mobilities, so the polymer phase is used to transport holes.
The nanoparticles transport electrons to the electrode.
The interfacial area between the polymer phase and the
nanoparticles needs to be large. This is achieved by dispersing the
particles throughout the polymer matrix. However, the nanoparticles need
to be interconnected to form percolation networks for electron
transport, which occurs by hopping events.
Efficiency is affected by aspect ratio, geometry, and volume
fraction of the nanoparticles. Nanoparticle structures include
nanocrystals, nanorods, and hyperbranched structures.
Figure 3 contains a picture of each structure. Different structures
change the conversion efficiency by effecting nanoparticle dispersion in
the polymer and providing pathways for electron transport.
The nanoparticle phase is required to provide a pathway for the
electrons to reach the electrode. By using nanorods instead of
nanocrystals, the hopping event from one crystal to another can be
avoided.
Fabrication methods include mixing the two materials in a
solution and spin-coating it onto a substrate, and solvent evaporation
(sol-gel). Most of these methods do not involve high-temperature
processing. Annealing increases order in the polymer phase, increasing
conductivity. However, annealing for too long causes the polymer domain
size to increase, eventually making it larger than the exciton diffusion
length, and possibly allowing some of the metal from the contact to
diffuse into the photoactive layer, reducing the efficiency of the
device.
Materials
Inorganic
semiconductor nanoparticles used in hybrid cells include CdSe (size
ranges from 6–20 nm), ZnO, TiO, and PbS. Common polymers used as photo
materials have extensive conjugation and are also hydrophobic. Their
efficiency as a photo-material is affected by the HOMO
level position and the ionization potential, which directly affects the
open circuit voltage and the stability in air. The most common polymers
used are P3HT (poly (3-hexylthiophene)), and M3H-PPV (poly[2-methoxy,
5-(2′-ethyl-hexyloxy)-p-phenylenevinylene)]). P3HT has a bandgap of 2.1
eV and M3H-PPV has a bandgap of ~2.4 eV. These values correspond with
the bandgap of CdSe, 2.10 eV. The electron affinity of CdSe ranges from
4.4 to 4.7 eV. When the polymer used is MEH-PPV, which has an electron
affinity of 3.0 eV, the difference between the electron affinities is
large enough to drive electron transfer from the CdSe to the polymer.
CdSe also has a high electron mobility (600 cm2·V−1·s−1).
Performance values
The
highest demonstrated efficiency is 3.2%, based upon a PCPDTBT polymer
donor and CdSe nanoparticle acceptor. The device exhibited a short
circuit current of 10.1 mA·cm−2, an open circuit voltage of .68 V, and a fill factor of .51.
Challenges
Hybrid
solar cells need increased efficiencies and stability over time before
commercialization is feasible. In comparison to the 2.4% of the CdSe-PPV
system, silicon photodevices have power conversion efficiencies greater
than 20%.
Problems include controlling the amount of nanoparticle
aggregation as the photolayer forms. The particles need to be dispersed
in order to maximize interface area, but need to aggregate to form
networks for electron transport. The network formation is sensitive to
the fabrication conditions. Dead end pathways can impede flow. A
possible solution is implementing ordered heterojunctions, where the
structure is well controlled.
The structures can undergo morphological changes over time,
namely phase separation. Eventually, the polymer domain size will be
greater than the carrier diffusion length, which lowers performance.
Even though the nanoparticle bandgap can be tuned, it needs to be
matched with the corresponding polymer. The 2.0 eV bandgap of CdSe is
larger than an ideal bandgap of 1.4 for absorbance of light.
The nanoparticles involved are typically colloids, which are
stabilized in solution by ligands. The ligands decrease device
efficiency because they serve as insulators which impede interaction
between the donor and nanoparticle acceptor as well as decreasing the
electron mobility. Some, but not complete success has been had by
exchanging the initial ligands for pyridine or another short chain
ligand.
Hybrid solar cells exhibit material properties inferior to those
of bulk silicon semiconductors. The carrier mobilities are much smaller
than that of silicon. Electron mobility in silicon is 1000 cm2·V−1·s−1, compared to 600 cm2·V−1·s−1 in CdSe, and less than 10 cm2·V−1·s−1 in other quantum dot materials. Hole mobility in MEH-PPV is 0.1 cm2·V−1·s−1, while in silicon it is 450 cm2·V−1·s−1.
Carbon nanotubes
Carbon nanotubes
(CNTs) have high electron conductivity, high thermal conductivity,
robustness, and flexibility. Field emission displays (FED), strain
sensors, and field effect transistors (FET) using CNTs have been
demonstrated.
Each application shows the potential of CNTs for nanoscale devices and
for flexible electronics applications. Photovoltaic applications have
also been explored for this material.
Mainly, CNTs have been used as either the photo-induced exciton
carrier transport medium impurity within a polymer-based photovoltaic
layer or as the photoactive (photon-electron conversion) layer. Metallic
CNT is preferred for the former application, while semiconducting CNT
is preferred for the later.
Efficient carrier transport medium
Device diagram for CNT as efficient carrier transport medium.
To increase the photovoltaic efficiency, electron-accepting
impurities must be added to the photoactive region. By incorporating
CNTs into the polymer, dissociation of the exciton pair can be
accomplished by the CNT matrix. The high surface area (~1600 m2/g)
of CNTs offers a good opportunity for exciton dissociation. The
separated carriers within the polymer-CNT matrix are transported by the
percolation pathways of adjacent CNTs, providing the means for high
carrier mobility and efficient charge transfer. The factors of
performance of CNT-polymer hybrid photovoltaics are low compared to
those of inorganic photovoltaics. SWNT in P3OT semiconductor polymer
demonstrated open circuit voltage (Voc) of below 0.94 V, with short circuit current (Isc) of 0.12 mA/cm2.
Metal nanoparticles may be applied to the exterior of CNTs to
increase the exciton separation efficiency. The metal provides a higher
electric field at the CNT-polymer interface, accelerating the exciton
carriers to transfer them more effectively to the CNT matrix. In this
case, Voc = 0.3396 V and Isc = 5.88 mA/cm2. The fill factor is 0.3876%, and the white light conversion factor 0.775%.
Photoactive matrix layer
CNT
may be used as a photovoltaic device not only as an add-in material to
increase carrier transport, but also as the photoactive layer itself.
The semiconducting single walled CNT (SWCNT) is a potentially
attractive material for photovoltaic applications for the unique
structural and electrical properties. SWCNT has high electric
conductivity (100 times that of copper) and shows ballistic carrier
transport, greatly decreasing carrier recombination. The bandgap of the SWCNT is inversely proportional to the tube diameter, which means that SWCNT may show multiple direct bandgaps matching the solar spectrum.
A strong built-in electric field in SWCNT for efficient
photogenerated electron-hole pair separation has been demonstrated by
using two asymmetrical metal electrodes with high and low work functions. The open circuit voltage (Voc) is 0.28 V, with short circuit current (Isc) 1.12 nA·cm−2 with an incident light source of 8.8 W·cm−2. The resulting white light conversion factor is 0.8%.
Challenges
Several
challenges must be addressed for CNT to be used in photovoltaic
applications. CNT degrades over time in an oxygen-rich environment. The
passivation layer required to prevent CNT oxidation may reduce the
optical transparency of the electrode region and lower the photovoltaic
efficiency.
Challenges as efficient carrier transport medium
Additional
challenges involve the dispersion of CNT within the polymer photoactive
layer. The CNT is required to be well dispersed within the polymer
matrix to form charge-transfer-efficient pathways between the excitons
and the electrode
Challenges as photoactive matrix layer
Challenges of CNT for the photoactive layer include its lack of capability to form a p-n junction,
due to the difficulty of doping certain segments of a CNT. (A p-n
junction creates an internal built-in potential, providing a pathway for
efficient carrier separation within the photovoltaic.) To overcome
this difficulty, energy band bending has been done by the use of two
electrodes of different work functions. A strong built-in electric field
covering the whole SWCNT channel is formed for high-efficiency carrier
separation. The oxidation issue with CNT is more critical for this
application. Oxidized CNTs have a tendency to become more metallic, and
so less useful as a photovoltaic material.
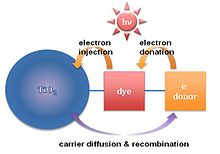
Dye-sensitized
Dye-sensitized solar cells
consists of a photo-sensitized anode, an electrolyte, and a
photo-electrochemical system. Hybrid solar cells based on dye-sensitized
solar cells are formed with inorganic materials (TiO2) and organic materials.
Materials
Hybrid
solar cells based on dye-sensitized solar cells are fabricated by
dye-absorbed inorganic materials and organic materials. TiO2
is the preferred inorganic material since this material is easy to
synthesize and acts as a n-type semiconductor due to the donor-like
oxygen vacancies. However, titania only absorbs a small fraction of the
UV spectrum. Molecular sensitizers (dye molecules) attached to the
semiconductor surface are used to collect a greater portion of the
spectrum. In the case of titania dye-sensitized solar cells, a photon
absorbed by a dye-sensitizer molecule layer induces electron injection
into the conduction band of titania, resulting in current flow. However,
short diffusion length (diffusivity, Dn≤10−4cm2/s)
in titania dye-sensitized solar cells decrease the solar-to-energy
conversion efficiency. To enhance diffusion length (or carrier
lifetime), a variety of organic materials are attached to the titania.
Fabrication scheme
Dye-sensitized photoelectrochemical cell (Grätzel cell)
Fig. 5. Schematic representation of electron-hole generation and recombination
TiO2 nanoparticles are synthesized in several tens of
nanometer scales (~100 nm). In order to make a photovoltaic cell,
molecular sensitizers (dye molecules) are attached to the titania
surface. The dye-absorbed titania is finally enclosed by a liquid
electrolyte. This type of dye-sensitized solar cell is also known as a
Grätzel cell. Dye-sensitized solar cell has a disadvantage of a short diffusion length. Recently, supermolecular or multifunctional sensitizers have been investigated so as to enhance carrier diffusion length. For example, a dye chromophore
has been modified by the addition of secondary electron donors.
Minority carriers (holes in this case) diffuse to the attached electron
donors to recombine. Therefore, electron-hole recombination is retarded
by the physical separation between the dye–cation moiety and the TiO2
surface, as shown in Fig. 5. Finally, this process raises the carrier
diffusion length, resulting in the increase of carrier lifetime.
Solid-state dye sensitized solar cell
Mesoporous materials contain pores with diameters between 2 and 50 nm. A dye-sensitized mesoporous film of TiO2
can be used for making photovoltaic cells and this solar cell is called
a ‘solid-state dye sensitized solar cell’. The pores in mesoporous TiO2
thin film are filled with a solid hole-conducting material such as
p-type semiconductors or organic hole conducting material. Replacing the
liquid electrolyte in Grätzel’s cells with a solid charge-transport
material can be beneficial. The process of electron-hole generation and
recombination is the same as Grätzel cells. Electrons are injected from
photoexcited dye into the conduction band of titania and holes are
transported by a solid charge transport electrolyte to an electrode.
Many organic materials have been tested to obtain a high solar-to-energy
conversion efficiency in dye synthesized solar cells based on
mesoporous titania thin film.
Efficiency factors
Efficiency factors demonstrated for dye-sensitized solar cells are:
| Parameters | Types of dye sensitized solar cells | |
|---|---|---|
| Grätzel cell | Solid-state | |
| Efficiency (%) | ~ 10–11 | ~ 4 |
| Voc (V) | ~ 0.7 | ~ 0.40 |
| Jsc (mA/cm2) | ~ 20 | ~ 9.10 |
| Fill factor | ~ 0.67 | ~ 0.6 |
Challenges
Liquid
organic electrolytes contain highly corrosive iodine, leading to
problems of leakage, sealing, handling, dye desorption, and maintenance.
Much attention is now focused on the electrolyte to address these
problems.
For solid-state dye sensitized solar cells, the first challenge
originates from disordered titania mesoporous structures. Mesoporous
titania structures should be fabricated with well-ordered titania
structures of uniform size (~ 10 nm). The second challenge comes from
developing the solid electrolyte, which is required to have these
properties:
- The electrolyte should be transparent to the visible spectrum (wide band gap).
- Fabrication should be possible for depositing the solid electrolyte without degrading the dye molecule layer on titania.
- The LUMO of the dye molecule should be higher than the conduction band of titania.
- Several p-type semiconductors tend to crystallize inside the mesoporous titania films, destroying the dye molecule-titania contact. Therefore, the solid electrolyte needs to be stable during operation.
Nanostructured inorganic — small molecules
In
2008, scientists were able to create a nanostructured lamellar
structure that provides an ideal design for bulk heterojunction solar
cells.
The observed structure is composed of ZnO and small, conducting organic
molecules, which co-assemble into alternating layers of organic and
inorganic components. This highly organized structure, which is
stabilized by π-π stacking between the organic molecules, allows for
conducting pathways in both the organic and inorganic layers. The
thicknesses of the layers (about 1–3 nm) are well within the exciton
diffusion length, which ideally minimizes recombination among charge
carriers. This structure also maximizes the interface between the
inorganic ZnO and the organic molecules, which enables a high
chromophore loading density within the structure. Due to the choice of
materials, this system is non-toxic and environmentally friendly, unlike
many other systems which use lead or cadmium.
Although this system has not yet been incorporated into a
photovoltaic device, preliminary photoconductivity measurements have
shown that this system exhibits among the highest values measured for
organic, hybrid, and amorphous silicon photoconductors, and so, offers
promise in creating efficient hybrid photovoltaic devices.