A selection of dye-sensitized solar cells.
A dye-sensitized solar cell (DSSC, DSC, DYSC or Grätzel cell) is a low-cost solar cell belonging to the group of thin film solar cells. It is based on a semiconductor formed between a photo-sensitized anode and an electrolyte, a photoelectrochemical system. The modern version of a dye solar cell, also known as the Grätzel cell, was originally co-invented in 1988 by Brian O'Regan and Michael Grätzel at UC Berkeley and this work was later developed by the aforementioned scientists at the École Polytechnique Fédérale de Lausanne until the publication of the first high efficiency DSSC in 1991. Michael Grätzel has been awarded the 2010 Millennium Technology Prize for this invention.
The DSSC has a number of attractive features; it is simple to
make using conventional roll-printing techniques, is semi-flexible and
semi-transparent which offers a variety of uses not applicable to
glass-based systems, and most of the materials used are low-cost. In
practice it has proven difficult to eliminate a number of expensive
materials, notably platinum and ruthenium, and the liquid electrolyte presents a serious challenge to making a cell suitable for use in all weather. Although its conversion efficiency is less than the best thin-film cells, in theory its price/performance ratio should be good enough to allow them to compete with fossil fuel electrical generation by achieving grid parity. Commercial applications, which were held up due to chemical stability problems,[6] are forecast in the European Union Photovoltaic Roadmap to significantly contribute to renewable electricity generation by 2020.
Current technology: semiconductor solar cells
In a traditional solid-state semiconductor, a solar cell is made from two doped crystals, one doped with n-type impurities (n-type semiconductor), which add additional free conduction band electrons, and the other doped with p-type impurities (p-type semiconductor), which add additional electron holes.
When placed in contact, some of the electrons in the n-type portion
flow into the p-type to "fill in" the missing electrons, also known as
electron holes. Eventually enough electrons will flow across the
boundary to equalize the Fermi levels of the two materials. The result is a region at the interface, the p-n junction,
where charge carriers are depleted and/or accumulated on each side of
the interface. In silicon, this transfer of electrons produces a potential barrier of about 0.6 to 0.7 V.
When placed in the sun, photons of the sunlight can excite electrons on the p-type side of the semiconductor, a process known as photoexcitation. In silicon, sunlight can provide enough energy to push an electron out of the lower-energy valence band into the higher-energy conduction band.
As the name implies, electrons in the conduction band are free to move
about the silicon. When a load is placed across the cell as a whole,
these electrons will flow out of the p-type side into the n-type side,
lose energy while moving through the external circuit, and then flow
back into the p-type material where they can once again re-combine with
the valence-band hole they left behind. In this way, sunlight creates an
electric current.
In any semiconductor, the band gap
means that only photons with that amount of energy, or more, will
contribute to producing a current. In the case of silicon, the majority
of visible light from red to violet has sufficient energy to make this
happen. Unfortunately higher energy photons, those at the blue and
violet end of the spectrum, have more than enough energy to cross the
band gap; although some of this extra energy is transferred into the
electrons, the majority of it is wasted as heat. Another issue is that
in order to have a reasonable chance of capturing a photon, the n-type
layer has to be fairly thick. This also increases the chance that a
freshly ejected electron will meet up with a previously created hole in
the material before reaching the p-n junction. These effects produce an
upper limit on the efficiency of silicon solar cells, currently around
12 to 15% for common modules and up to 25% for the best laboratory cells
(33.16% is the theoretical maximum efficiency for single band gap solar
cells).
By far the biggest problem with the conventional approach is
cost; solar cells require a relatively thick layer of doped silicon in
order to have reasonable photon capture rates, and silicon processing is
expensive. There have been a number of different approaches to reduce
this cost over the last decade, notably the thin-film
approaches, but to date they have seen limited application due to a
variety of practical problems. Another line of research has been to
dramatically improve efficiency through the multi-junction
approach, although these cells are very high cost and suitable only for
large commercial deployments. In general terms the types of cells
suitable for rooftop deployment have not changed significantly in
efficiency, although costs have dropped somewhat due to increased
supply.
Dye-sensitized solar cells
Type of cell made at the EPFL by Grätzel and O'Regan
Operation of a Grätzel cell.
In the late 1960s it was discovered that illuminated organic dyes can
generate electricity at oxide electrodes in electrochemical cells.
In an effort to understand and simulate the primary processes in
photosynthesis the phenomenon was studied at the University of
California at Berkeley with chlorophyll extracted from spinach
(bio-mimetic or bionic approach).
On the basis of such experiments electric power generation via the dye
sensitization solar cell (DSSC) principle was demonstrated and discussed
in 1972.
The instability of the dye solar cell was identified as a main
challenge. Its efficiency could, during the following two decades, be
improved by optimizing the porosity of the electrode prepared from fine
oxide powder, but the instability remained a problem.
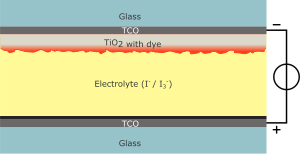
A modern DSSC is composed of a porous layer of titanium dioxide nanoparticles, covered with a molecular dye that absorbs sunlight, like the chlorophyll in green leaves. The titanium dioxide is immersed under an electrolyte solution, above which is a platinum-based catalyst. As in a conventional alkaline battery, an anode (the titanium dioxide) and a cathode (the platinum) are placed on either side of a liquid conductor (the electrolyte).
Sunlight passes through the transparent electrode into the dye
layer where it can excite electrons that then flow into the titanium
dioxide. The electrons flow toward the transparent electrode where they
are collected for powering a load. After flowing through the external
circuit, they are re-introduced into the cell on a metal electrode on
the back, flowing into the electrolyte. The electrolyte then transports
the electrons back to the dye molecules.
Dye-sensitized solar cells separate the two functions provided by
silicon in a traditional cell design. Normally the silicon acts as both
the source of photoelectrons, as well as providing the electric field
to separate the charges and create a current. In the dye-sensitized
solar cell, the bulk of the semiconductor is used solely for charge
transport, the photoelectrons are provided from a separate photosensitive dye. Charge separation occurs at the surfaces between the dye, semiconductor and electrolyte.
The dye molecules are quite small (nanometer sized), so in order
to capture a reasonable amount of the incoming light the layer of dye
molecules needs to be made fairly thick, much thicker than the molecules
themselves. To address this problem, a nanomaterial is used as a
scaffold to hold large numbers of the dye molecules in a 3-D matrix,
increasing the number of molecules for any given surface area of cell.
In existing designs, this scaffolding is provided by the semiconductor
material, which serves double-duty.
Construction
In the case of the original Grätzel and O'Regan design, the cell has 3 primary parts. On top is a transparent anode made of fluoride-doped tin dioxide (SnO2:F) deposited on the back of a (typically glass) plate. On the back of this conductive plate is a thin layer of titanium dioxide (TiO2), which forms into a highly porous structure with an extremely high surface area. The (TiO2) is chemically bound by a process called sintering. TiO2 only absorbs a small fraction of the solar photons (those in the UV). The plate is then immersed in a mixture of a photosensitive ruthenium-polypyridine dye (also called molecular sensitizers) and a solvent. After soaking the film in the dye solution, a thin layer of the dye is left covalently bonded to the surface of the TiO2. The bond is either an ester, chelating, or bidentate bridging linkage.
A separate plate is then made with a thin layer of the iodide electrolyte spread over a conductive sheet, typically platinum
metal. The two plates are then joined and sealed together to prevent
the electrolyte from leaking. The construction is simple enough that
there are hobby kits available to hand-construct them.
Although they use a number of "advanced" materials, these are
inexpensive compared to the silicon needed for normal cells because they
require no expensive manufacturing steps. TiO2, for instance, is already widely used as a paint base.
One of the efficient DSSCs devices uses ruthenium-based molecular dye, e.g. [Ru(4,4'-dicarboxy-2,2'-bipyridine)2(NCS)2]
(N3), that is bound to a photoanode via carboxylate moieties. The
photoanode consists of 12 μm thick film of transparent 10–20 nm diameter
TiO2 nanoparticles covered with a 4 μm thick film of much
larger (400 nm diameter) particles that scatter photons back into the
transparent film. The excited dye rapidly injects an electron into the
TiO2 after light absorption. The injected electron diffuses
through the sintered particle network to be collected at the front side
transparent conducting oxide (TCO) electrode, while the dye is
regenerated via reduction by a redox shuttle, I3/I, dissolved in a solution. Diffusion of the oxidized form of the shuttle to the counter electrode completes the circuit.
Mechanism of DSSCs
The main processes that occur in a DSSC to convert photons(light) to current are:- The incident photon is absorbed by Ru complex photosensitizers adsorbed on the TiO2 surface.
- The photosensitizers are excited from the ground state (S) to the excited state (S∗). The excited electrons are injected into the conduction band of the TiO2 electrode. This results in the oxidation of the photosensitizer (S+).
-
S+hν→S∗
-
- The injected electrons in the conduction band of TiO2 are transported between TiO2 nanoparticles with diffusion toward the back contact (TCO). And the electrons finally reach the counter electrode through the circuit.
- The oxidized photosensitizer (S+) accepts electrons from the I− ion redox mediator leading to regeneration of the ground state (S), and two I−-Ions are oxidized to elementary Iodine which reacts with I− to the oxidized state, I3−.
-
S++e−→S
-
- The oxidized redox mediator, I3−, diffuses toward the counter electrode and then it is reduced to I− ions.
-
I3−+2e−→3I−
-
The efficiency of a DSSC depends on four energy levels of the
component: the excited state (approximately LUMO) and the ground state
(HOMO) of the photosensitizer, the Fermi level of the TiO2 electrode and the redox potential of the mediator (I−/I3−) in the electrolyte.
Nanoplant-like morphology
In DSSC, electrodes consisted of sintered semiconducting nanoparticles, mainly TiO2
or ZnO. These nanoparticle DSSCs rely on trap-limited diffusion through
the semiconductor nanoparticles for the electron transport. This limits
the device efficiency since it is a slow transport mechanism.
Recombination is more likely to occur at longer wavelengths of
radiation. Moreover, sintering of nanoparticles requires a high
temperature of about 450 °C, which restricts the fabrication of these
cells to robust, rigid solid substrates. It has been proven that there
is an increase in the efficiency of DSSC, if the sintered nanoparticle
electrode is replaced by a specially designed electrode possessing an
exotic 'nanoplant-like' morphology.
Operation
Sunlight enters the cell through the transparent SnO2:F top contact, striking the dye on the surface of the TiO2.
Photons striking the dye with enough energy to be absorbed create an
excited state of the dye, from which an electron can be "injected"
directly into the conduction band of the TiO2. From there it moves by diffusion (as a result of an electron concentration gradient) to the clear anode on top.
Meanwhile, the dye molecule has lost an electron and the molecule
will decompose if another electron is not provided. The dye strips one
from iodide in electrolyte below the TiO2, oxidizing it into triiodide.
This reaction occurs quite quickly compared to the time that it takes
for the injected electron to recombine with the oxidized dye molecule,
preventing this recombination reaction that would effectively short-circuit the solar cell.
The triiodide then recovers its missing electron by mechanically diffusing to the bottom of the cell, where the counter electrode re-introduces the electrons after flowing through the external circuit.
Efficiency
Several important measures are used to characterize solar cells. The
most obvious is the total amount of electrical power produced for a
given amount of solar power shining on the cell. Expressed as a
percentage, this is known as the solar conversion efficiency. Electrical power is the product of current and voltage, so the maximum values for these measurements are important as well, Jsc and Voc
respectively. Finally, in order to understand the underlying physics,
the "quantum efficiency" is used to compare the chance that one photon
(of a particular energy) will create one electron.
In quantum efficiency
terms, DSSCs are extremely efficient. Due to their "depth" in the
nanostructure there is a very high chance that a photon will be
absorbed, and the dyes are very effective at converting them to
electrons. Most of the small losses that do exist in DSSC's are due to
conduction losses in the TiO2 and the clear electrode, or
optical losses in the front electrode. The overall quantum efficiency
for green light is about 90%, with the "lost" 10% being largely
accounted for by the optical losses in the top electrode. The quantum
efficiency of traditional designs vary, depending on their thickness,
but are about the same as the DSSC.
In theory, the maximum voltage generated by such a cell is simply the difference between the (quasi-)Fermi level of the TiO2 and the redox potential of the electrolyte, about 0.7 V under solar illumination conditions (Voc).
That is, if an illuminated DSSC is connected to a voltmeter in an "open
circuit", it would read about 0.7 V. In terms of voltage, DSSCs offer
slightly higher Voc than silicon, about 0.7 V compared to
0.6 V. This is a fairly small difference, so real-world differences are
dominated by current production, Jsc.
Although the dye is highly efficient at converting absorbed photons into free electrons in the TiO2,
only photons absorbed by the dye ultimately produce current. The rate
of photon absorption depends upon the absorption spectrum of the
sensitized TiO2 layer and upon the solar flux spectrum. The
overlap between these two spectra determines the maximum possible
photocurrent. Typically used dye molecules generally have poorer
absorption in the red part of the spectrum compared to silicon, which
means that fewer of the photons in sunlight are usable for current
generation. These factors limit the current generated by a DSSC, for
comparison, a traditional silicon-based solar cell offers about 35 mA/cm2, whereas current DSSCs offer about 20 mA/cm2.
Overall peak power conversion efficiency for current DSSCs is about 11%. Current record for prototypes lies at 15%.
Degradation
DSSCs degrade when exposed to ultraviolet
radiation. In 2014 air infiltration of the commonly-used amorphous
Spiro-MeOTAD hole-transport layer was identified as the primary cause of
the degradation, rather than oxidation. The damage could be avoided by
the addition of an appropriate barrier.
The barrier layer may include UV stabilizers and/or UV absorbing luminescent chromophores (which emit at longer wavelengths which may be reabsorbed by the dye) and antioxidants to protect and improve the efficiency of the cell.
Advantages
DSSCs are currently the most efficient third-generation
(2005 Basic Research Solar Energy Utilization 16) solar technology
available. Other thin-film technologies are typically between 5% and
13%, and traditional low-cost commercial silicon panels operate between
14% and 17%. This makes DSSCs attractive as a replacement for existing
technologies in "low density" applications like rooftop solar
collectors, where the mechanical robustness and light weight of the
glass-less collector is a major advantage. They may not be as attractive
for large-scale deployments where higher-cost higher-efficiency cells
are more viable, but even small increases in the DSSC conversion
efficiency might make them suitable for some of these roles as well.
There is another area where DSSCs are particularly attractive. The process of injecting an electron directly into the TiO2
is qualitatively different from that occurring in a traditional cell,
where the electron is "promoted" within the original crystal. In theory,
given low rates of production, the high-energy electron in the silicon
could re-combine with its own hole, giving off a photon (or other form
of energy) which does not result in current being generated. Although
this particular case may not be common, it is fairly easy for an
electron generated by another atom to combine with a hole left behind in
a previous photoexcitation.
In comparison, the injection process used in the DSSC does not introduce a hole in the TiO2,
only an extra electron. Although it is energetically possible for the
electron to recombine back into the dye, the rate at which this occurs
is quite slow compared to the rate that the dye regains an electron from
the surrounding electrolyte. Recombination directly from the TiO2 to species in the electrolyte is also possible although, again, for optimized devices this reaction is rather slow. On the contrary, electron transfer from the platinum coated electrode to species in the electrolyte is necessarily very fast.
As a result of these favorable "differential kinetics", DSSCs
work even in low-light conditions. DSSCs are therefore able to work
under cloudy skies and non-direct sunlight, whereas traditional designs
would suffer a "cutout" at some lower limit of illumination, when charge
carrier mobility is low and recombination becomes a major issue. The
cutoff is so low they are even being proposed for indoor use, collecting
energy for small devices from the lights in the house.
A practical advantage which DSSCs share with most thin-film
technologies, is that the cell's mechanical robustness indirectly leads
to higher efficiencies at higher temperatures. In any semiconductor,
increasing temperature will promote some electrons into the conduction
band "mechanically". The fragility of traditional silicon cells requires
them to be protected from the elements, typically by encasing them in a
glass box similar to a greenhouse,
with a metal backing for strength. Such systems suffer noticeable
decreases in efficiency as the cells heat up internally. DSSCs are
normally built with only a thin layer of conductive plastic on the front
layer, allowing them to radiate away heat much easier, and therefore
operate at lower internal temperatures.
Disadvantages
The
major disadvantage to the DSSC design is the use of the liquid
electrolyte, which has temperature stability problems. At low
temperatures the electrolyte can freeze, ending power production and
potentially leading to physical damage. Higher temperatures cause the
liquid to expand, making sealing the panels a serious problem. Another
disadvantage is that costly ruthenium (dye), platinum (catalyst) and
conducting glass or plastic (contact) are needed to produce a DSSC. A
third major drawback is that the electrolyte solution contains volatile organic compounds (or VOC's),
solvents which must be carefully sealed as they are hazardous to human
health and the environment. This, along with the fact that the solvents
permeate plastics, has precluded large-scale outdoor application and
integration into flexible structure.
Replacing the liquid electrolyte with a solid has been a major
ongoing field of research. Recent experiments using solidified melted
salts have shown some promise, but currently suffer from higher
degradation during continued operation, and are not flexible.
Photocathodes and tandem cells
Dye
sensitised solar cells operate as a photoanode (n-DSC), where
photocurrent result from electron injection by the sensitized dye.
Photocathodes (p-DSCs) operate in an inverse mode compared to the
conventional n-DSC, where dye-excitation is followed by rapid electron
transfer from a p-type semiconductor to the dye (dye-sensitized hole
injection, instead of electron injection). Such p-DSCs and n-DSCs can be
combined to construct tandem solar cells (pn-DSCs) and the theoretical
efficiency of tandem DSCs is well beyond that of single-junction DSCs.
A standard tandem cell consists of one n-DSC and one p-DSC in a
simple sandwich configuration with an intermediate electrolyte layer.
n-DSC and p-DSC are connected in series, which implies that the
resulting photocurrent will be controlled by the weakest photoelectrode,
whereas photovoltages are additive. Thus, photocurrent matching is very
important for the construction of highly efficient tandem pn-DSCs.
However, unlike n-DSCs, fast charge recombination following
dye-sensitized hole injection usually resulted in low photocurrents in
p-DSC and thus hampered the efficiency of the overall device.
Researchers have found that using dyes comprising a perylenemonoimide
(PMI) as the acceptor and an oligothiophene coupled to triphenylamine
as the donor greatly improve the performance of p-DSC by reducing charge
recombination rate following dye-sensitized hole injection. The
researchers constructed a tandem DSC device with NiO on the p-DSC side
and TiO2 on the n-DSC side. Photocurrent matching was achieved through adjustment of NiO and TiO2
film thicknesses to control the optical absorptions and therefore match
the photocurrents of both electrodes. The energy conversion efficiency
of the device is 1.91%, which exceeds the efficiency of its individual
components, but is still much lower than that of high performance n-DSC
devices (6%–11%). The results are still promising since the tandem DSC
was in itself rudimentary. The dramatic improvement in performance in
p-DSC can eventually lead to tandem devices with much greater efficiency
than lone n-DSCs.
Development
"Black Dye", an anionic Ru-terpyridine complex
The dyes used in early experimental cells (circa 1995) were sensitive
only in the high-frequency end of the solar spectrum, in the UV and
blue. Newer versions were quickly introduced (circa 1999) that had much
wider frequency response, notably "triscarboxy-ruthenium terpyridine"
[Ru(4,4',4"-(COOH)3-terpy)(NCS)3], which is efficient right into the low-frequency range of red and IR light. The wide spectral response results in the dye having a deep brown-black color, and is referred to simply as "black dye".
The dyes have an excellent chance of converting a photon into an
electron, originally around 80% but improving to almost perfect
conversion in more recent dyes, the overall efficiency is about 90%,
with the "lost" 10% being largely accounted for by the optical losses in
top electrode.
A solar cell must be capable of producing electricity for at least twenty years, without a significant decrease in efficiency.
The "black dye" system was subjected to 50 million cycles, the
equivalent of ten years' exposure to the sun in Switzerland. No
discernible performance decrease was observed. However the dye is
subject to breakdown in high-light situations. Over the last decade an
extensive research program has been carried out to address these
concerns. The newer dyes included 1-ethyl-3 methylimidazolium
tetrocyanoborate [EMIB(CN)4] which is extremely light- and temperature-stable, copper-diselenium [Cu(In,GA)Se2] which offers higher conversion efficiencies, and others with varying special-purpose properties.
DSSCs are still at the start of their development cycle.
Efficiency gains are possible and have recently started more widespread
study. These include the use of quantum dots
for conversion of higher-energy (higher frequency) light into multiple
electrons, using solid-state electrolytes for better temperature
response, and changing the doping of the TiO2 to better match it with the electrolyte being used.
New developments
2003
A
group of researchers at the Swiss Federal Institute of Technology has
reportedly increased the thermostability of DSC by using amphiphilic
ruthenium sensitizer in conjunction with quasi-solid-state gel
electrolyte. The stability of the device matches that of a conventional
inorganic silicon-based solar cell. The cell sustained heating for 1,000
h at 80 °C.
The group has previously prepared a ruthenium amphiphilic dye Z-907 (cis-Ru(H2dcbpy)(dnbpy)(NCS)2, where the ligand H2dcbpy
is 4,4′-dicarboxylic acid-2,2′-bipyridine and dnbpy is
4,4′-dinonyl-2,2′-bipyridine) to increase dye tolerance to water in the
electrolytes. In addition, the group also prepared a quasi-solid-state
gel electrolyte with a 3-methoxypropionitrile (MPN)-based liquid
electrolyte that was solidified by a photochemically stable fluorine
polymer, polyvinylidenefluoride-co-hexafluoropropylene (PVDF-HFP).
The use of the amphiphilic Z-907 dye in conjunction with the
polymer gel electrolyte in DSC achieved an energy conversion efficiency
of 6.1%. More importantly, the device was stable under thermal stress
and soaking with light. The high conversion efficiency of the cell was
sustained after heating for 1,000 h at 80 °C, maintaining 94% of its
initial value. After
accelerated testing in a solar simulator for 1,000 h of light-soaking at 55 °C (100 mW cm−2)
the efficiency had decreased by less than 5% for cells covered with an
ultraviolet absorbing polymer film. These results are well within the
limit for that of traditional inorganic silicon solar cells.
The enhanced performance may arise from a decrease in solvent
permeation across the sealant due to the application of the polymer gel
electrolyte. The polymer gel electrolyte is quasi-solid at room
temperature, and becomes a viscous liquid (viscosity: 4.34 mPa·s) at
80 °C compared with the traditional liquid electrolyte (viscosity: 0.91
mPa·s). The much improved stabilities of the device under both thermal
stress and soaking with light has never before been seen in DSCs, and
they match the durability criteria applied to solar cells for outdoor
use, which makes these devices viable for practical application.
2006
The first successful solid-hybrid dye-sensitized solar cells were reported.
To improve electron transport in these solar cells, while
maintaining the high surface area needed for dye adsorption, two
researchers have designed alternate semiconductor morphologies, such as
arrays of nanowires and a combination of nanowires and nanoparticles,
to provide a direct path to the electrode via the semiconductor
conduction band. Such structures may provide a means to improve the
quantum efficiency of DSSCs in the red region of the spectrum, where
their performance is currently limited.
On August 2006, to prove the chemical and thermal robustness of
the 1-ethyl-3 methylimidazolium tetracyanoborate solar cell, the
researchers subjected the devices to heating at 80 °C in the dark for
1000 hours, followed by light soaking at 60 °C for 1000 hours. After dark heating
and light soaking, 90% of the initial photovoltaic efficiency was
maintained – the first time such excellent thermal stability has been
observed for a liquid electrolyte that exhibits such a high conversion
efficiency. Contrary to silicon solar cells,
whose performance declines with increasing temperature, the
dye-sensitized solar-cell devices were only negligibly influenced when
increasing the operating temperature from ambient to 60 °C.
April 2007
Wayne Campbell at Massey University, New Zealand, has experimented with a wide variety of organic dyes based on porphyrin. In nature, porphyrin is the basic building block of the hemoproteins, which include chlorophyll in plants and hemoglobin in animals. He reports efficiency on the order of 5.6% using these low-cost dyes.
June 2008
An article published in Nature Materials
demonstrated cell efficiencies of 8.2% using a new solvent-free liquid
redox electrolyte consisting of a melt of three salts, as an alternative
to using organic solvents as an electrolyte solution. Although the
efficiency with this electrolyte is less than the 11% being delivered
using the existing iodine-based solutions, the team is confident the
efficiency can be improved.
2009
A group of researchers at Georgia Tech made dye-sensitized solar cells with a higher effective surface area by wrapping the cells around a quartz optical fiber. The researchers removed the cladding from optical fibers, grew zinc oxide nanowires along the surface, treated them with dye molecules, surrounded the fibers by an electrolyte
and a metal film that carries electrons off the fiber. The cells are
six times more efficient than a zinc oxide cell with the same surface
area.
Photons bounce inside the fiber as they travel, so there are more
chances to interact with the solar cell and produce more current. These
devices only collect light at the tips, but future fiber cells could be
made to absorb light along the entire length of the fiber, which would
require a coating that is conductive as well as transparent. Max Shtein of the University of Michigan said a sun-tracking system would not be necessary for such cells, and would work on cloudy days when light is diffuse.
2010
Researchers at the École Polytechnique Fédérale de Lausanne and at the Université du Québec à Montréal claim to have overcome two of the DSC's major issues:
- "New molecules" have been created for the electrolyte, resulting in a liquid or gel that is transparent and non-corrosive, which can increase the photovoltage and improve the cell's output and stability.
- At the cathode, platinum was replaced by cobalt sulfide, which is far less expensive, more efficient, more stable and easier to produce in the laboratory.
2011
Dyesol and Tata Steel Europe
announced in June the development of the world's largest dye sensitized
photovoltaic module, printed onto steel in a continuous line.
Dyesol and CSIRO
announced in October a Successful Completion of Second Milestone in
Joint Dyesol / CSIRO Project.
Dyesol Director Gordon Thompson said, "The materials developed during
this joint collaboration have the potential to significantly advance the
commercialisation of DSC in a range of applications where performance
and stability are essential requirements.
Dyesol is extremely encouraged by the breakthroughs in the chemistry
allowing the production of the target molecules. This creates a path to
the immediate commercial utilisation of these new materials."
Dyesol and Tata Steel Europe
announced in November the targeted development of Grid Parity
Competitive BIPV solar steel that does not require government subsidised
feed in tariffs. TATA-Dyesol "Solar Steel" Roofing is currently being
installed on the Sustainable Building Envelope Centre (SBEC) in Shotton,
Wales.
2012
Northwestern University researchers announced
a solution to a primary problem of DSSCs, that of difficulties in using
and containing the liquid electrolyte and the consequent relatively
short useful life of the device. This is achieved through the use of nanotechnology
and the conversion of the liquid electrolyte to a solid. The current
efficiency is about half that of silicon cells, but the cells are
lightweight and potentially of much lower cost to produce.
2013
During the
last 5–10 years, a new kind of DSSC has been developed — the solid state
dye-sensitized solar cell. In this case the liquid electrolyte is
replaced by one of several solid hole conducting materials. From 2009 to
2013 the efficiency of Solid State DSSCs has dramatically increased
from 4% to 15%. Michael Grätzel announced the fabrication of Solid State
DSSCs with 15.0% efficiency, reached by the means of a hybrid perovskite CH3NH3PbI3 dye, subsequently deposited from the separated solutions of CH3NH3I and PbI2.
The first architectural integration was demonstrated at EPFL's
new convention center in partnership with Romande Energie. The total
surface is 300 m2, in 1400 modules of 50 cm x 35 cm. Designed by artists Daniel Schlaepfer and Catherine Bolle.
2018
Researchers have investigated the role of surface plasmon resonances present on gold nanorods
in the performance of dye-sensitized solar cells. They found that with
an increase nanorod concentration, the light absorption grew linearly;
however, charge extraction was also dependent on the concentration.
With an optimized concentration, they found that the overall power
conversion efficiency improved from 5.31 to 8.86% for Y123
dye-sensitized solar cells.
The synthesis of one-dimensional TiO2 nanostructures directly on fluorine-doped tin oxide glass substrates was successful demonstrated via a two-stop solvothermal reaction. Additionally, through a TiO2 sol treatment, the performance of the dual TiO2 nanowire cells was enhanced, reaching a power conversion efficiency of 7.65%.
Stainless steel based counter-electrodes for DSSCs have been
reported which further reduce cost compared to conventional platinum
based counter electrode and are suitable for outdoor application.
Researchers from EPFL have advanced the DSSCs based on copper complexes redox electrolytes, which have achieved 13.1% efficiency under standard AM1.5G, 100 mW/cm2 conditions and record 32% efficiency under 1000 lux of indoor light.
Market introduction
Several commercial providers are promising availability of DSCs in the near future:
- Dyesol officially opened its new manufacturing facilities in Queanbeyan Australia on 7 October 2008. It has subsequently announced partnerships with Tata Steel (TATA-Dyesol) and Pilkington Glass (Dyetec-Solar) for the development and large scale manufacture of DSC BIPV. Dyesol has also entered working relationships with Merck, Umicore, CSIRO, Japanese Ministry of Economy and Trade, Singapore Aerospace Manufacturing and a joint Venture with TIMO Korea (Dyesol-TIMO).
- Solaronix, a Swiss company specialized in the production of DSC materials since 1993, has extended their premises in 2010 to host a manufacturing pilot line of DSC modules.[57]
- SolarPrint was founded in Ireland in 2008 by Dr. Mazhar Bari, Andre Fernon and Roy Horgan. SolarPrint was the first Ireland-based commercial entity involved in the manufacturing of PV technology. SolarPrint's innovation was the solution to the solvent-based electrolyte which to date has prohibited the mass commercialization of DSSC. The company went into receivership in 2014 and was wound up.
- G24innovations founded in 2006, based in Cardiff, South Wales, UK. On 17 October 2007, claimed the production of the first commercial grade dye sensitized thin films.
- Sony Corporation has developed dye-sensitized solar cells with an energy conversion efficiency of 10%, a level seen as necessary for commercial use.
- Tasnee Enters Strategic Investment Agreement with Dyesol.
- H.Glass was founded 2011 in Switzerland. H.Glass has put enormous efforts to create industrial process for the DSSC technologie - the first results where shown at the EXPO 2015 in Milano at the Austrian Pavilion. The milestone for DSSC is the Science Tower in Austria - it is the largest installation of DSSC in the world - carried out by SFL technologies.


![{\displaystyle {\ce {S^{.}->[{} \atop {\ce {TiO2}}]{S+}+e-}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/4a8461f7deace264f9b77a6e9f2d2a67e729f5fa)