Elements heavier than nickel are comparatively rare owing to the
decline with atomic weight of their nuclear binding energies per
nucleon, but they too are created in part within supernovae. Of greatest
interest historically has been their synthesis by rapid capture of neutrons during the r-process, reflecting the common belief that supernova cores are likely to provide the necessary conditions. But see the r-process below for a recently discovered alternative. The r-process
isotopes are roughly a 100,000 times less abundant than the primary
chemical elements fused in supernova shells above. Furthermore, other
nucleosynthesis processes in supernovae are thought to also be
responsible for some nucleosynthesis of other heavy elements, notably,
the proton capture process known as the rp-process, the slow capture of neutrons (s-process) in the Helium-burning shells and in the carbon-burning shells of massive stars, and a photodisintegration process known as the γ-process
(gamma-process). The latter synthesizes the lightest, most
neutron-poor, isotopes of the elements heavier than iron from
preexisting heavier isotopes.
History
The
theory that nucleosynthesis of the chemical elements occurred primarily
during advanced evolution of massive stars was first proposed by Hoyle
in 1954, in which he predicted the existence of the excited state in the 12C nucleus that enables the triple-alpha process
to burn resonantly, enabling it to heat the helium cores of stars while
synthesizing massive quantities of carbon and oxygen; and he introduced
the thermonuclear sequels of carbon-burning synthesizing Ne, Mg and Na and of oxygen-burning synthesizing Si, Al and S. Hoyle could not yet convincingly discern how silicon burning
would happen, although he foresaw that it must be the final core fusion
prior to operation of his thermal-equilibrium picture of iron formation.
He also predicted that the collapse of the evolved cores of massive
stars was "inevitable" owing to their increasing rate of energy loss by
neutrinos. This work was so advanced relative to the state of
astrophysics that it was hard to digest. Hoyle's 1954 theory fell into
obscurity for decades after the more-famous B2FH paper was published in 1957 and, surprisingly, did not include Hoyle's original description of nucleosynthesis in massive stars. Donald D. Clayton has attributed the obscurity also to Hoyle's 1954 paper describing its key equation only in words, and a lack of careful review by Hoyle of the B2FH draft by coauthors who had themselves not adequately studied Hoyle's paper. During his 1955 discussions in Cambridge with his coauthors in preparation of the B2FH first draft in 1956 in Pasadena, Hoyle's modesty had inhibited him from emphasizing to them the great achievements of his 1954 theory.
Thirteen years after the B2FH paper, W. D. Arnett and colleagues
demonstrated that the final burning in the passing shock wave launched
by collapse of the core could synthesize non-alpha-particle isotopes
more effectively than hydrostatic burning could,
suggesting that explosive nucleosynthesis is an essential component of
supernova nucleosynthesis. A shock wave rebounded from matter collapsing
onto the dense core, if strong enough to lead to mass ejection of the
mantle of supernovae, would necessarily be strong enough to provide the
sudden heating of the shells of massive stars needed for explosive
thermonuclear burning within the mantle. Understanding how that shock
wave can reach the mantle in the face of continuing infall onto the
shock that became the theoretical difficulty. Supernova observations assured that it must occur.
Era of Computer Models
The
papers of Hoyle (1946) and Hoyle (1954) and of B2FH (1957) were written
by those scientists before the advent of the age of computers. They
relied on hand calculations, deep thought, physical intuition, and
familiarity with details of nuclear physics. Brilliant as these founding
papers were, a cultural disconnect soon emerged with a younger
generation of scientists who began to construct computer programs that would eventually yield numerical answers for the advanced evolution of stars and the nucleosynthesis within them.
Most of this new generation never digested Hoyle (1954) carefully and
in any case forgot what they had read in their focus on the immense task
of computerizing massive stars. They usually did not cite Hoyle (1954),
but they did cite B2FH as a needed default citation for stellar
nucleosynthesis. This computer cultural revolution began in late 1960s.
The upshot in regard to the puzzling confusion over Hoyle and B2FH that
followed was made possible by the B2FH review's failure to describe
Hoyle’s picture. Understandable was the feeling by the new generation of
themselves discovering the correct picture that Hoyle had presented,
albeit with huge numerical details that Hoyle could not provide. The
computer models of massive stars demonstrated that core burning in
massive stars occurred in smaller cores than the previous burning phase
had. This shrinking of successive cores yielded an onion shell model
of the sequence of burning phases, a shell model that was necessary for
Hoyle's 1954 picture to work as simultaneous ejection of the abundances
from each burning phase. Understanding this computer cultural
revolution takes one far in understanding why Hoyle (1954) was forgotten
and B2FH appeared to have been the work that founded stellar
nucleosynthesis, as many even claimed. The field of working astronomers
became devoted to B2FH owing to that paper's citation of about 100
research papers by astronomers showing evidence of abundance changes in
stars owing to nuclear reactions. Such abundance alterations, which were
visible at the telescopes, became confused with Hoyle's goal of
understanding the origin of the huge interstellar abundances of the
elements.
Cause
A supernova is a violent explosion of a star that occurs under two principal scenarios. The first is that a white dwarf star,
which is the remnant of a low-mass star that has exhausted its nuclear
fuel, undergoes a thermonuclear explosion after its mass is increased
beyond its Chandrasekhar limit by accreting nuclear-fuel mass from a more diffuse companion star (usually a red giant)
with which it is in binary orbit. The second, and about threefold more
common, scenario occurs when a massive star (12–35 times more massive
than the sun), usually a supergiant at the critical time, reaches nickel-56 in its core nuclear fusion
(or burning) processes. Without exothermic energy from fusion, the core
of the pre-supernova massive star loses heat needed for pressure
support, and collapses owing to the strong gravitational pull. The
energy transfer from the core collapse causes the supernova display. The nickel-56 isotope has one of the largest binding energies per nucleon of all isotopes, and is therefore the last isotope whose synthesis during core silicon burning releases energy by nuclear fusion, exothermically. The binding energy per nucleon declines for atomic weights heavier than A = 56,
ending fusion's history of supplying thermal energy to the star. The
thermal energy released when the infalling supernova mantle hits the
semi-solid core is very large, about 1053 ergs, about a
hundred times the energy released by the supernova as the kinetic energy
of its ejected mass. Dozens of research papers have been published in
the attempt to describe the hydrodynamics of how that small one percent
of the in falling energy is transmitted to the overlying mantle in the
face of continuous infall onto the core. That uncertainty remains in the
full description of core-collapse supernovae.
Nuclear fusion reactions that produce elements heavier than iron absorb nuclear energy and are said to be endothermic
reactions. When such reactions dominate, the internal temperature that
supports the star's outer layers drops. Because the outer envelope is no
longer sufficiently supported by the radiation pressure, the star's
gravity pulls its mantle rapidly inward. As the star collapses, this
mantle collides violently with the growing incompressible stellar core,
which has a density almost as great as an atomic nucleus, producing a
shockwave that rebounds outward through the unfused material of the
outer shell. The increase of temperature by the passage of that
shockwave is sufficient to induce fusion in that material, often called explosive nucleosynthesis.
The energy deposited by the shockwave somehow leads to the star's
explosion, dispersing fusing matter in the mantle above the core into interstellar space.
Silicon burning
After a star completes the oxygen burning process, its core is composed primarily of silicon and sulfur.
If it has sufficiently high mass, it further contracts until its core
reaches temperatures in the range of 2.7–3.5 billion Kelvin (230–300 keV).
At these temperatures, silicon and other isotopes suffer photoejection
of nucleons by energetic thermal photons (γ) ejecting especially alpha
particles (4He).
The nuclear process of silicon burning differs from earlier fusion
stages of nucleosynthesis in that it entails a balance between
alpha-particle captures and their inverse photo ejection which
establishes abundances of all alpha-particle elements in the following
sequence in which each alpha particle capture shown is opposed by its
inverse reaction, namely, photo ejection of an alpha particle by the
abundant thermal photons:
28Si + 4He ⇌ 32S + γ; 32S + 4He ⇌ 36Ar + γ; 36Ar + 4He ⇌ 40Ca + γ; 40Ca + 4He ⇌ 44Ti + γ; 44Ti + 4He ⇌ 48Cr + γ; 48Cr + 4He ⇌ 52Fe + γ; 52Fe + 4He ⇌ 56Ni + γ; 56Ni + 4He ⇌ 60Zn + γ.
The alpha-particle nuclei 44Ti and those more massive in
the final five reactions listed are all radioactive, but they decay
after their ejection in supernova explosions into abundant isotopes of
Ca, Ti, Cr, Fe and Ni. This post-supernova radioactivity became of great
importance for the emergence of gamma-ray-line astronomy.
In these physical circumstances of rapid opposing reactions,
namely alpha-particle capture and photo ejection of alpha particles, the
abundances are not determined by alpha-particle-capture cross sections;
rather they are determined by the values that the abundances must
assume in order to balance the speeds of the rapid opposing-reaction
currents. Each abundance takes on a stationary value that achieves that balance. This picture is called nuclear quasiequilibrium. Many computer calculations, for example,
using the numerical rates of each reaction and of their reverse
reactions have demonstrated that quasiequilibrium is not exact but does
characterize well the computed abundances. Thus the quasiequilibrium
picture presents a comprehensible picture of what actually happens. It
also fills in an uncertainty in Hoyle's 1954 theory. The
quasiequilibrium buildup shuts off after 56Ni because the
alpha-particle captures become slower whereas the photo ejections from
heavier nuclei become faster. Non-alpha-particle nuclei also
participate, using a host of reactions similar to 36Ar + neutron ⇌ 37Ar
+ photon and its inverse which set the stationary abundances of the
non-alpha-particle isotopes, where the free densities of protons and
neutrons are also established by the quasiequilibrium. However, the
abundance of free neutrons is also proportional to the excess of
neutrons over protons in the composition of the massive star; therefore
the abundance of 37Ar, using it as an example, is greater in
ejecta from recent massive stars than it was from those in early stars
of only H and He; therefore 37Cl, to which 37Ar
decays after the nucleosynthesis, is called a "secondary isotope". The
silicon burning in the star progresses through a temporal sequence of
such nuclear quasiequilibria in which the abundance of 28Si slowly declines and that of 56Ni slowly increases. This amounts to a nuclear abundance change 2 28Si ≫ 56Ni,
which may be thought of as silicon burning into nickel in the nuclear
sense. In interest of economy the photodisintegration rearrangement and
the nuclear quasiequilibrium that it achieves is referred to as silicon burning.
The entire silicon-burning sequence lasts about one day in the core of a contracting massive star and stops after 56Ni
has become the dominant abundance. The final explosive burning caused
when the supernova shock passes through the silicon-burning shell lasts
only seconds, but its roughly 50% increase in the temperature causes
furious nuclear burning, which becomes the major contributor to
nucleosynthesis in the mass range 28–60. The star can no longer release energy via nuclear fusion because a nucleus with 56 nucleons has the lowest mass per nucleon of all the elements in the sequence. The next step up in the alpha-particle chain would be 60Zn, which has slightly more mass per nucleon and thus is less thermodynamically favorable. 56Ni (which has 28 protons) has a half-life of 6.02 days and decays via β+ decay to 56Co (27 protons), which in turn has a half-life of 77.3 days as it decays to 56Fe (26 protons). However, only minutes are available for the 56Ni to decay within the core of a massive star. This establishes 56Ni as the most abundant of the radioactive nuclei created in this way. Its radioactivity energizes the late supernova light curve and creates the pathbreaking opportunity for gamma-ray-line astronomy. Clayton and Meyer have recently generalized this process still further by what they have named the secondary supernova machine,
attributing the increasing radioactivity that energizes late supernova
displays to the storage of increasing Coulomb energy within the
quasiequilibrium nuclei called out above as the quasiequilibria shift
from primarily 28Si to primarily 56Ni. The visible displays are powered by the decay of that excess Coulomb energy.
During this phase of the core contraction, the potential energy
of gravitational compression heats the interior to roughly three billion
degrees K, which briefly maintains pressure support and opposes rapid
core contraction. However, since no additional heat energy can be
generated via new fusion reactions, the final unopposed contraction
rapidly accelerates into a collapse lasting only a few seconds. The
central portion of the star is now crushed into either a neutron star or, if the star is massive enough, a black hole. The outer layers of the star are blown off in an explosion triggered by the outward moving supernova shock, known as a Type II supernova
whose displays last days to months. The escaping portion of the
supernova core may initially contain a large density of free neutrons,
which may synthesize, in about one second while inside the star, roughly
half of the elements in the universe that are heavier than iron via a
rapid neutron-capture mechanism known as the r-process. See below.
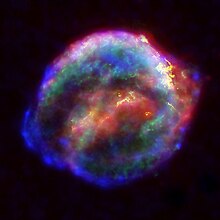
Nuclides synthesized
Composite image of Kepler's supernova from pictures by the Spitzer Space Telescope, Hubble Space Telescope, and Chandra X-ray Observatory.
Stars with initial masses less than about eight times the sun never
develop a core large enough to collapse and they eventually lose their
atmospheres to become white dwarfs, stable cooling spheres of carbon
supported by the pressure of degenerate electrons. Nucleosynthesis
within those lighter stars is therefore limited to nuclides
that were fused in material located above the final white dwarf. This
limits their modest yields returned to interstellar gas to carbon-13 and
nitrogen-14, and to isotopes heavier than iron by slow capture of
neutrons (the s-process).
A significant minority of white dwarfs will nonetheless explode,
however, because they formed in a binary orbit with a giant companion
star that loses mass to the stronger gravitational field of the white
dwarf, which then grows past its Chandrasekhar limit and explodes as a
Type Ia supernova, synthesizing about a solar mass of radioactive 56Ni
isotopes. Its radioactive decay to iron keeps Type Ia optically very
bright for weeks and creates more than half of all iron in the universe.
Virtually all of the remainder of stellar nucleosynthesis occurs,
however, in more frequent stars that are massive enough to end as Type II supernovae.
In the presupernova massive star this includes helium burning, carbon
burning, oxygen burning and silicon burning. Much of that yield may
never leave the star but instead disappears into its collapsed core. The
yield that is ejected is substantially fused in last-second explosive
burning caused by the shock wave launched by core collapse.
Prior to core collapse, fusion of elements between silicon and iron
occurs only in the largest of stars, and then in limited amounts. Thus
the nucleosynthesis of the abundant primary elements
defined as those that could be synthesized in stars of initially only
hydrogen and helium (left by the Big Bang), is substantially limited to
core-collapse supernova nucleosynthesis.
The r-process
A version of the periodic table indicating the main origin of elements found on Earth. All elements past plutonium (element 94) are manmade.
During supernova nucleosynthesis, the r-process creates very neutron-rich heavy isotopes, which decay after the event to the first stable isotope,
thereby creating the neutron-rich stable isotopes of all heavy
elements. This neutron capture process occurs in high neutron density
with high temperature conditions. In the r-process, any heavy nuclei are bombarded with a large neutron flux to form highly unstable neutron rich nuclei which very rapidly undergo beta decay to form more stable nuclei with higher atomic number and the same atomic mass. The neutron density is extremely high, about 1022-24 neutrons per cubic centimeter. First calculation of an evolving r-process, showing the evolution of calculated results with time, also suggested that the r-process abundances are a superposition of differing neutron fluences. Small fluence produces the first r-process abundance peak near atomic weight A = 130 but no actinides, whereas large fluence produces the actinides uranium and thorium but no longer contains the A = 130
abundance peak. These processes occur in a fraction of a second to a
few seconds, depending on details. Hundreds of subsequent papers
published have utilized this time-dependent approach. The only modern
nearby supernova, 1987A, has not revealed r-process enrichments. Modern thinking is that the r-process
yield may be ejected from some supernovae but swallowed up in others as
part of the residual neutron star or black hole.
Entirely new astronomical data about the r-process was discovered in 2017 when the LIGO and Virgo gravitational-wave observatories discovered a merger of two neutron stars that had previously been orbiting one another
That can happen when both massive stars in orbit with one another
become core-collapse supernovae, leaving neutron-star remnants. Everyone
could "hear" the replay of the increasing orbital frequency as the
orbit became smaller and faster owing to energy loss by gravitational
waves. The localization on the sky of the source of those gravitational
waves radiated by that orbital collapse and merger of the two neutron
stars, creating a black hole, but with significant spun off mass of
highly neutronized matter, enabled several teams to discover and study the remaining optical counterpart of the merger, finding spectroscopic evidence of r-process
material thrown off by the merging neutron stars. The bulk of this
material seems to consist of two types: hot blue masses of highly
radioactive r-process matter of lower-mass-range heavy nuclei (A < 140) and cooler red masses of higher mass-number r-process nuclei (A > 140)
rich in lanthanides (such as uranium, thorium, californium etc.). When
released from the huge internal pressure of the neutron star, these
neutralized ejecta expand and radiate detected optical light for about a
week. Such duration of luminosity would not be possible without heating
by internal radioactive decay, which is provided by r-process nuclei near their waiting points. Two distinct mass regions (A < 140 and A > 140) for the r-process yields have been known since the first time dependent calculations of the r-process. Because of these spectroscopic features it has been argued that r-process nucleosynthesis in the Milky Way may have been primarily ejecta from neutron-star mergers rather than from supernovae. These results offer a new possibility for clarifying six decades of uncertainty over the site of origin of r-process nuclei. Confirming relevance of this discovery by gravitational-wave astronomy to the r-process is the radiogenic power from radioactive decay of r-process nuclei that maintains the visibility of these spun off r-process
fragments. Otherwise they would dim quickly. Unquestionably this
discovery raised support for such mergers being the main sources of the r-process nuclei rather than core-collapse supernovae; but that debate continues.