Differences in the concentrations of ions on opposite sides of a cellular membrane lead to a voltage called the membrane potential.
Typical values of membrane potential are in the range –40 mV to –70
mV. Many ions have a concentration gradient across the membrane,
including potassium (K+), which is at a high concentration inside and a low concentration outside the membrane. Sodium (Na+) and chloride (Cl−) ions are at high concentrations in the extracellular region, and low concentrations in the intracellular regions. These concentration gradients provide the potential energy
to drive the formation of the membrane potential. This voltage is
established when the membrane has permeability to one or more ions. In
the simplest case, illustrated here, if the membrane is selectively
permeable to potassium, these positively charged ions can diffuse down
the concentration gradient to the outside of the cell, leaving behind
uncompensated negative charges. This separation of charges is what
causes the membrane potential. Note that the system as a whole is
electro-neutral. The uncompensated positive charges outside the cell,
and the uncompensated negative charges inside the cell, physically line
up on the membrane surface and attract each other across the lipid bilayer.
Thus, the membrane potential is physically located only in the
immediate vicinity of the membrane. It is the separation of these
charges across the membrane that is the basis of the membrane voltage.
Note also that this diagram is only an approximation of the ionic
contributions to the membrane potential. Other ions including sodium,
chloride, calcium, and others play a more minor role, even though they
have strong concentration gradients, because they have more limited
permeability than potassium. Key: Blue pentagons – sodium ions; Purple squares – potassium ions; Yellow circles – chloride ions; Orange rectangles – membrane-impermeable anions (these arise from a variety of sources including proteins). The large purple structure with an arrow represents a transmembrane potassium channel and the direction of net potassium movement.
Membrane potential (also transmembrane potential or membrane voltage) is the difference in electric potential between the interior and the exterior of a biological cell. With respect to the exterior of the cell, typical values of membrane potential, normally given in millivolts, range from –40 mV to –80 mV.
All animal cells are surrounded by a membrane composed of a lipid bilayer with proteins embedded in it. The membrane serves as both an insulator and a diffusion barrier to the movement of ions. Transmembrane proteins, also known as ion transporter or ion pump proteins, actively push ions across the membrane and establish concentration gradients across the membrane, and ion channels
allow ions to move across the membrane down those concentration
gradients. Ion pumps and ion channels are electrically equivalent to a
set of batteries and resistors inserted in the membrane, and therefore create a voltage between the two sides of the membrane.
Almost all plasma membranes have an electrical potential across
them, with the inside usually negative with respect to the outside.
The membrane potential has two basic functions. First, it allows a cell
to function as a battery, providing power to operate a variety of
"molecular devices" embedded in the membrane. Second, in electrically excitable cells such as neurons and muscle cells,
it is used for transmitting signals between different parts of a cell.
Signals are generated by opening or closing of ion channels at one point
in the membrane, producing a local change in the membrane potential.
This change in the electric field can be quickly affected by either
adjacent or more distant ion channels in the membrane. Those ion
channels can then open or close as a result of the potential change,
reproducing the signal.
In non-excitable cells, and in excitable cells in their baseline
states, the membrane potential is held at a relatively stable value,
called the resting potential.
For neurons, typical values of the resting potential range from –70 to
–80 millivolts; that is, the interior of a cell has a negative baseline
voltage of a bit less than one-tenth of a volt. The opening and closing
of ion channels can induce a departure from the resting potential. This
is called a depolarization if the interior voltage becomes less negative (say from –70 mV to –60 mV), or a hyperpolarization
if the interior voltage becomes more negative (say from –70 mV to –80
mV). In excitable cells, a sufficiently large depolarization can evoke
an action potential,
in which the membrane potential changes rapidly and significantly for a
short time (on the order of 1 to 100 milliseconds), often reversing its
polarity. Action potentials are generated by the activation of certain
voltage-gated ion channels.
In neurons, the factors that influence the membrane potential are
diverse. They include numerous types of ion channels, some of which are
chemically gated and some of which are voltage-gated. Because
voltage-gated ion channels are controlled by the membrane potential,
while the membrane potential itself is influenced by these same ion
channels, feedback loops that allow for complex temporal dynamics arise,
including oscillations and regenerative events such as action
potentials.
Physical basis
The
membrane potential in a cell derives ultimately from two factors:
electrical force and diffusion. Electrical force arises from the mutual
attraction between particles with opposite electrical charges (positive
and negative) and the mutual repulsion between particles with the same
type of charge (both positive or both negative). Diffusion arises from
the statistical tendency of particles to redistribute from regions where
they are highly concentrated to regions where the concentration is low.
Voltage
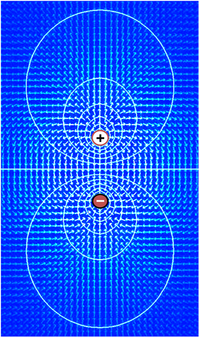
Electric
field (arrows) and contours of constant voltage created by a pair of
oppositely charged objects. The electric field is at right angles to the
voltage contours, and the field is strongest where the spacing between
contours is the smallest.
Voltage, which is synonymous with difference in electrical potential, is the ability to drive an electric current across a resistance. Indeed, the simplest definition of a voltage is given by Ohm's law:
V=IR, where V is voltage, I is current and R is resistance. If a
voltage source such as a battery is placed in an electrical circuit, the
higher the voltage of the source the greater the amount of current that
it will drive across the available resistance. The functional
significance of voltage lies only in potential differences
between two points in a circuit. The idea of a voltage at a single point
is meaningless. It is conventional in electronics to assign a voltage
of zero to some arbitrarily chosen element of the circuit, and then
assign voltages for other elements measured relative to that zero point.
There is no significance in which element is chosen as the zero
point—the function of a circuit depends only on the differences not on
voltages per se. However, in most cases and by convention, the
zero level is most often assigned to the portion of a circuit that is in
contact with ground.
The same principle applies to voltage in cell biology. In
electrically active tissue, the potential difference between any two
points can be measured by inserting an electrode at each point, for
example one inside and one outside the cell, and connecting both
electrodes to the leads of what is in essence a specialized voltmeter.
By convention, the zero potential value is assigned to the outside of
the cell and the sign of the potential difference between the outside
and the inside is determined by the potential of the inside relative to
the outside zero.
In mathematical terms, the definition of voltage begins with the concept of an electric field E, a vector field assigning a magnitude and direction to each point in space. In many situations, the electric field is a conservative field, which means that it can be expressed as the gradient of a scalar function V, that is, E = –∇V. This scalar field V
is referred to as the voltage distribution. Note that the definition
allows for an arbitrary constant of integration—this is why absolute
values of voltage are not meaningful. In general, electric fields can
be treated as conservative only if magnetic fields do not significantly
influence them, but this condition usually applies well to biological
tissue.
Because the electric field is the gradient of the voltage
distribution, rapid changes in voltage within a small region imply a
strong electric field; on the converse, if the voltage remains
approximately the same over a large region, the electric fields in that
region must be weak. A strong electric field, equivalent to a strong
voltage gradient, implies that a strong force is exerted on any charged
particles that lie within the region.
Ions and the forces driving their motion
Ions
(pink circles) will flow across a membrane from the higher
concentration to the lower concentration (down a concentration
gradient), causing a current. However, this creates a voltage across the
membrane that opposes the ions' motion. When this voltage reaches the
equilibrium value, the two balance and the flow of ions stops.
Electrical signals within biological organisms are, in general, driven by ions. The most important cations for the action potential are sodium (Na+) and potassium (K+). Both of these are monovalent cations that carry a single positive charge. Action potentials can also involve calcium (Ca2+), which is a divalent cation that carries a double positive charge. The chloride anion (Cl−) plays a major role in the action potentials of some algae, but plays a negligible role in the action potentials of most animals.
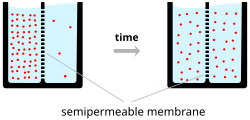
Ions cross the cell membrane under two influences: diffusion and electric fields.
A simple example wherein two solutions—A and B—are separated by a
porous barrier illustrates that diffusion will ensure that they will
eventually mix into equal solutions. This mixing occurs because of the
difference in their concentrations. The region with high concentration
will diffuse out toward the region with low concentration. To extend the
example, let solution A have 30 sodium ions and 30 chloride ions. Also,
let solution B have only 20 sodium ions and 20 chloride ions. Assuming
the barrier allows both types of ions to travel through it, then a
steady state will be reached whereby both solutions have 25 sodium ions
and 25 chloride ions. If, however, the porous barrier is selective to
which ions are let through, then diffusion alone will not determine the
resulting solution. Returning to the previous example, let's now
construct a barrier that is permeable only to sodium ions. Now, only
sodium is allowed to diffuse cross the barrier from its higher
concentration in solution A to the lower concentration in solution B.
This will result in a greater accumulation of sodium ions than chloride
ions in solution B and a lesser number of sodium ions than chloride ions
in solution A.
This means that there is a net positive charge in solution B from
the higher concentration of positively charged sodium ions than
negatively charged chloride ions. Likewise, there is a net negative
charge in solution A from the greater concentration of negative chloride
ions than positive sodium ions. Since opposite charges attract and like
charges repel, the ions are now also influenced by electrical fields as
well as forces of diffusion. Therefore, positive sodium ions will be
less likely to travel to the now-more-positive B solution and remain in
the now-more-negative A solution. The point at which the forces of the
electric fields completely counteract the force due to diffusion is
called the equilibrium potential. At this point, the net flow of the
specific ion (in this case sodium) is zero.
Plasma membranes
The cell membrane, also called the plasma membrane or plasmalemma, is a semipermeable
lipid bilayer common to all living cells. It contains a variety of
biological molecules, primarily proteins and lipids, which are involved
in a vast array of cellular processes.
Every animal cell is enclosed in a plasma membrane, which has the structure of a lipid bilayer
with many types of large molecules embedded in it. Because it is made
of lipid molecules, the plasma membrane intrinsically has a high
electrical resistivity, in other words a low intrinsic permeability to
ions. However, some of the molecules embedded in the membrane are
capable either of actively transporting ions from one side of the
membrane to the other or of providing channels through which they can
move.
In electrical terminology, the plasma membrane functions as a combined resistor and capacitor.
Resistance arises from the fact that the membrane impedes the movement
of charges across it. Capacitance arises from the fact that the lipid
bilayer is so thin that an accumulation of charged particles on one side
gives rise to an electrical force that pulls oppositely charged
particles toward the other side. The capacitance of the membrane is
relatively unaffected by the molecules that are embedded in it, so it
has a more or less invariant value estimated at about 2 µF/cm2
(the total capacitance of a patch of membrane is proportional to its
area). The conductance of a pure lipid bilayer is so low, on the other
hand, that in biological situations it is always dominated by the
conductance of alternative pathways provided by embedded molecules.
Thus, the capacitance of the membrane is more or less fixed, but the
resistance is highly variable.
The thickness of a plasma membrane is estimated to be about 7-8
nanometers. Because the membrane is so thin, it does not take a very
large transmembrane voltage to create a strong electric field within it.
Typical membrane potentials in animal cells are on the order of 100
millivolts (that is, one tenth of a volt), but calculations show that
this generates an electric field close to the maximum that the membrane
can sustain—it has been calculated that a voltage difference much larger
than 200 millivolts could cause dielectric breakdown, that is, arcing across the membrane.
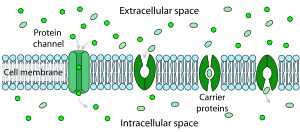
Facilitated diffusion and transport
Facilitated diffusion in cell membranes, showing ion channels and carrier proteins
The resistance of a pure lipid bilayer to the passage of ions across
it is very high, but structures embedded in the membrane can greatly
enhance ion movement, either actively or passively, via mechanisms called facilitated transport and facilitated diffusion. The two types of structure that play the largest roles are ion channels and ion pumps,
both usually formed from assemblages of protein molecules. Ion channels
provide passageways through which ions can move. In most cases, an ion
channel is permeable only to specific types of ions (for example, sodium
and potassium but not chloride or calcium), and sometimes the
permeability varies depending on the direction of ion movement. Ion
pumps, also known as ion transporters or carrier proteins, actively
transport specific types of ions from one side of the membrane to the
other, sometimes using energy derived from metabolic processes to do so.
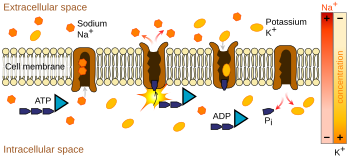
Ion pumps
The sodium-potassium pump uses energy derived from ATP to exchange sodium for potassium ions across the membrane.
Ion pumps are integral membrane proteins that carry out active transport, i.e., use cellular energy (ATP) to "pump" the ions against their concentration gradient.
Such ion pumps take in ions from one side of the membrane (decreasing
its concentration there) and release them on the other side (increasing
its concentration there).
The ion pump most relevant to the action potential is the sodium–potassium pump, which transports three sodium ions out of the cell and two potassium ions in. As a consequence, the concentration of potassium ions K+
inside the neuron is roughly 20-fold larger than the outside
concentration, whereas the sodium concentration outside is roughly
ninefold larger than inside. In a similar manner, other ions have different concentrations inside and outside the neuron, such as calcium, chloride and magnesium.
If the numbers of each type of ion were equal, the
sodium–potassium pump would be electrically neutral, but, because of the
three-for-two exchange, it gives a net movement of one positive charge
from intracellular to extracellular for each cycle, thereby contributing
to a positive voltage difference. The pump has three effects: (1) it
makes the sodium concentration high in the extracellular space and low
in the intracellular space; (2) it makes the potassium concentration
high in the intracellular space and low in the extracellular space; (3)
it gives the intracellular space a negative voltage with respect to the
extracellular space.
The sodium-potassium pump is relatively slow in operation. If a
cell were initialized with equal concentrations of sodium and potassium
everywhere, it would take hours for the pump to establish equilibrium.
The pump operates constantly, but becomes progressively less efficient
as the concentrations of sodium and potassium available for pumping are
reduced.
Ion pumps influence the action potential only by establishing the
relative ratio of intracellular and extracellular ion concentrations.
The action potential involves mainly the opening and closing of ion
channels not ion pumps. If the ion pumps are turned off by removing
their energy source, or by adding an inhibitor such as ouabain, the axon can still fire hundreds of thousands of action potentials before their amplitudes begin to decay significantly. In particular, ion pumps play no significant role in the repolarization of the membrane after an action potential.
Another functionally important ion pump is the sodium-calcium exchanger. This pump operates in a conceptually similar way to the sodium-potassium pump, except that in each cycle it exchanges three Na+ from the extracellular space for one Ca++
from the intracellular space. Because the net flow of charge is inward,
this pump runs "downhill", in effect, and therefore does not require
any energy source except the membrane voltage. Its most important effect
is to pump calcium outward—it also allows an inward flow of sodium,
thereby counteracting the sodium-potassium pump, but, because overall
sodium and potassium concentrations are much higher than calcium
concentrations, this effect is relatively unimportant. The net result of
the sodium-calcium exchanger is that in the resting state,
intracellular calcium concentrations become very low.
Ion channels
Despite the small differences in their radii, ions rarely go through the "wrong" channel. For example, sodium or calcium ions rarely pass through a potassium channel.
Ion channels are integral membrane proteins
with a pore through which ions can travel between extracellular space
and cell interior. Most channels are specific (selective) for one ion;
for example, most potassium channels are characterized by 1000:1
selectivity ratio for potassium over sodium, though potassium and sodium
ions have the same charge and differ only slightly in their radius. The
channel pore is typically so small that ions must pass through it in
single-file order.
Channel pores can be either open or closed for ion passage, although
a number of channels demonstrate various sub-conductance levels. When a
channel is open, ions permeate through the channel pore down the
transmembrane concentration gradient for that particular ion. Rate of
ionic flow through the channel, i.e. single-channel current amplitude,
is determined by the maximum channel conductance and electrochemical
driving force for that ion, which is the difference between the
instantaneous value of the membrane potential and the value of the reversal potential.
Depiction
of the open potassium channel, with the potassium ion shown in purple
in the middle, and hydrogen atoms omitted. When the channel is closed,
the passage is blocked.
A channel may have several different states (corresponding to different conformations
of the protein), but each such state is either open or closed. In
general, closed states correspond either to a contraction of the
pore—making it impassable to the ion—or to a separate part of the
protein, stoppering the pore. For example, the voltage-dependent sodium
channel undergoes inactivation, in which a portion of the protein swings into the pore, sealing it. This inactivation shuts off the sodium current and plays a critical role in the action potential.
Ion channels can be classified by how they respond to their environment. For example, the ion channels involved in the action potential are voltage-sensitive channels; they open and close in response to the voltage across the membrane. Ligand-gated channels form another important class; these ion channels open and close in response to the binding of a ligand molecule, such as a neurotransmitter. Other ion channels open and close with mechanical forces. Still other ion channels—such as those of sensory neurons—open and close in response to other stimuli, such as light, temperature or pressure.
Leakage channels
Leakage channels
are the simplest type of ion channel, in that their permeability is
more or less constant. The types of leakage channels that have the
greatest significance in neurons are potassium and chloride channels. It
should be noted that even these are not perfectly constant in their
properties: First, most of them are voltage-dependent in the sense that
they conduct better in one direction than the other (in other words,
they are rectifiers);
second, some of them are capable of being shut off by chemical ligands
even though they do not require ligands in order to operate.
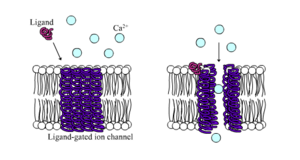
Ligand-gated channels
Ligand-gated calcium channel in closed and open states
Ligand-gated ion channels
are channels whose permeability is greatly increased when some type of
chemical ligand binds to the protein structure. Animal cells contain
hundreds, if not thousands, of types of these. A large subset function
as neurotransmitter receptors—they occur at postsynaptic sites, and the chemical ligand that gates them is released by the presynaptic axon terminal. One example of this type is the AMPA receptor, a receptor for the neurotransmitter glutamate that when activated allows passage of sodium and potassium ions. Another example is the GABAA receptor, a receptor for the neurotransmitter GABA that when activated allows passage of chloride ions.
Neurotransmitter receptors are activated by ligands that appear
in the extracellular area, but there are other types of ligand-gated
channels that are controlled by interactions on the intracellular side.
Voltage-dependent channels
Voltage-gated ion channels, also known as voltage dependent ion channels,
are channels whose permeability is influenced by the membrane
potential. They form another very large group, with each member having a
particular ion selectivity and a particular voltage dependence. Many
are also time-dependent—in other words, they do not respond immediately
to a voltage change but only after a delay.
One of the most important members of this group is a type of
voltage-gated sodium channel that underlies action potentials—these are
sometimes called Hodgkin-Huxley sodium channels because they were initially characterized by Alan Lloyd Hodgkin and Andrew Huxley
in their Nobel Prize-winning studies of the physiology of the action
potential. The channel is closed at the resting voltage level, but opens
abruptly when the voltage exceeds a certain threshold, allowing a large
influx of sodium ions that produces a very rapid change in the membrane
potential. Recovery from an action potential is partly dependent on a
type of voltage-gated potassium channel that is closed at the resting
voltage level but opens as a consequence of the large voltage change
produced during the action potential.
Reversal potential
The reversal potential (or equilibrium potential)
of an ion is the value of transmembrane voltage at which diffusive and
electrical forces counterbalance, so that there is no net ion flow
across the membrane. This means that the transmembrane voltage exactly
opposes the force of diffusion of the ion, such that the net current of
the ion across the membrane is zero and unchanging. The reversal
potential is important because it gives the voltage that acts on
channels permeable to that ion—in other words, it gives the voltage that
the ion concentration gradient generates when it acts as a battery.
The equilibrium potential of a particular ion is usually designated by the notation Eion.The equilibrium potential for any ion can be calculated using the Nernst equation. For example, reversal potential for potassium ions will be as follows:
where
- Eeq,K+ is the equilibrium potential for potassium, measured in volts
- R is the universal gas constant, equal to 8.314 joules·K−1·mol−1
- T is the absolute temperature, measured in kelvins (= K = degrees Celsius + 273.15)
- z is the number of elementary charges of the ion in question involved in the reaction
- F is the Faraday constant, equal to 96,485 coulombs·mol−1 or J·V−1·mol−1
- [K+]o is the extracellular concentration of potassium, measured in mol·m−3 or mmol·l−1
- [K+]i is the intracellular concentration of potassium
Even if two different ions have the same charge (i.e., K+ and Na+),
they can still have very different equilibrium potentials, provided
their outside and/or inside concentrations differ. Take, for example,
the equilibrium potentials of potassium and sodium in neurons. The
potassium equilibrium potential EK is −84 mV with 5 mM potassium outside and 140 mM inside. On the other hand, the sodium equilibrium potential, ENa, is approximately +66 mV with approximately 12 mM sodium inside and 140 mM outside.
Changes to membrane potential during development
A neuron’s resting membrane potential actually changes during the development
of an organism. In order for a neuron to eventually adopt its full
adult function, its potential must be tightly regulated during
development. As an organism progresses through development the resting
membrane potential becomes more negative. Glial cells are also differentiating and proliferating as development progresses in the brain. The addition of these glial cells increases the organism’s ability to regulate extracellular potassium. The drop in extracellular potassium can lead to a decrease in membrane potential of 35 mV.
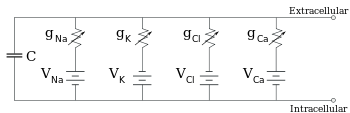
Equivalent circuit
Equivalent
circuit for a patch of membrane, consisting of a fixed capacitance in
parallel with four pathways each containing a battery in series with a
variable conductance
Electrophysiologists model the effects of ionic concentration
differences, ion channels, and membrane capacitance in terms of an equivalent circuit,
which is intended to represent the electrical properties of a small
patch of membrane. The equivalent circuit consists of a capacitor in
parallel with four pathways each consisting of a battery in series with a
variable conductance. The capacitance is determined by the properties
of the lipid bilayer, and is taken to be fixed. Each of the four
parallel pathways comes from one of the principal ions, sodium,
potassium, chloride, and calcium. The voltage of each ionic pathway is
determined by the concentrations of the ion on each side of the
membrane; see the Reversal potential
section above. The conductance of each ionic pathway at any point in
time is determined by the states of all the ion channels that are
potentially permeable to that ion, including leakage channels,
ligand-gated channels, and voltage-gated ion channels.
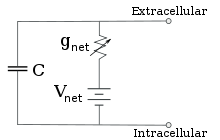
Reduced circuit obtained by combining the ion-specific pathways using the Goldman equation
For fixed ion concentrations and fixed values of ion channel
conductance, the equivalent circuit can be further reduced, using the Goldman equation
as described below, to a circuit containing a capacitance in parallel
with a battery and conductance. In electrical terms, this is a type of RC circuit
(resistance-capacitance circuit), and its electrical properties are
very simple. Starting from any initial state, the current flowing
across either the conductance or the capacitance decays with an
exponential time course, with a time constant of τ = RC, where C is the capacitance of the membrane patch, and R = 1/gnet
is the net resistance. For realistic situations, the time constant
usually lies in the 1—100 millisecond range. In most cases, changes in
the conductance of ion channels occur on a faster time scale, so an RC
circuit is not a good approximation; however, the differential equation
used to model a membrane patch is commonly a modified version of the RC
circuit equation.
Resting potential
When the membrane potential of a cell goes for a long period of time without changing significantly, it is referred to as a resting potential
or resting voltage. This term is used for the membrane potential of
non-excitable cells, but also for the membrane potential of excitable
cells in the absence of excitation. In excitable cells, the other
possible states are graded membrane potentials (of variable amplitude),
and action potentials, which are large, all-or-nothing rises in membrane
potential that usually follow a fixed time course. Excitable cells
include neurons, muscle cells, and some secretory cells in glands.
Even in other types of cells, however, the membrane voltage can
undergo changes in response to environmental or intracellular stimuli.
For example, depolarization of the plasma membrane appears to be an
important step in programmed cell death.
The interactions that generate the resting potential are modeled by the Goldman equation.
This is similar in form to the Nernst equation shown above, in that it
is based on the charges of the ions in question, as well as the
difference between their inside and outside concentrations. However, it
also takes into consideration the relative permeability of the plasma
membrane to each ion in question.
The three ions that appear in this equation are potassium (K+), sodium (Na+), and chloride (Cl−). Calcium is omitted, but can be added to deal with situations in which it plays a significant role.
Being an anion, the chloride terms are treated differently from the
cation terms; the intracellular concentration is in the numerator, and
the extracellular concentration in the denominator, which is reversed
from the cation terms. Pi stands for the relative permeability of the ion type i.
In essence, the Goldman formula expresses the membrane potential
as a weighted average of the reversal potentials for the individual ion
types, weighted by permeability. (Although the membrane potential
changes about 100 mV during an action potential, the concentrations of
ions inside and outside the cell do not change significantly. They
remain close to their respective concentrations when then membrane is at
resting potential.) In most animal cells, the permeability to potassium
is much higher in the resting state than the permeability to sodium.
As a consequence, the resting potential is usually close to the
potassium reversal potential.
The permeability to chloride can be high enough to be significant,
but, unlike the other ions, chloride is not actively pumped, and
therefore equilibrates at a reversal potential very close to the resting
potential determined by the other ions.
Values of resting membrane potential in most animal cells usually
vary between the potassium reversal potential (usually around -80 mV)
and around -40 mV. The resting potential in excitable cells (capable of
producing action potentials) is usually near -60 mV—more depolarized
voltages would lead to spontaneous generation of action potentials.
Immature or undifferentiated cells show highly variable values of
resting voltage, usually significantly more positive than in
differentiated cells.
In such cells, the resting potential value correlates with the degree
of differentiation: undifferentiated cells in some cases may not show
any transmembrane voltage difference at all.
Maintenance of the resting potential can be metabolically costly
for a cell because of its requirement for active pumping of ions to
counteract losses due to leakage channels. The cost is highest when the
cell function requires an especially depolarized value of membrane
voltage. For example, the resting potential in daylight-adapted blowfly (Calliphora vicina) photoreceptors can be as high as -30 mV.
This elevated membrane potential allows the cells to respond very
rapidly to visual inputs; the cost is that maintenance of the resting
potential may consume more than 20% of overall cellular ATP.
On the other hand, the high resting potential in undifferentiated
cells can be a metabolic advantage. This apparent paradox is resolved
by examination of the origin of that resting potential.
Little-differentiated cells are characterized by extremely high input
resistance,
which implies that few leakage channels are present at this stage of
cell life. As an apparent result, potassium permeability becomes similar
to that for sodium ions, which places resting potential in-between the
reversal potentials for sodium and potassium as discussed above. The
reduced leakage currents also mean there is little need for active
pumping in order to compensate, therefore low metabolic cost.
Graded potentials
As
explained above, the potential at any point in a cell's membrane is
determined by the ion concentration differences between the
intracellular and extracellular areas, and by the permeability of the
membrane to each type of ion. The ion concentrations do not normally
change very quickly (with the exception of Ca2+, where the
baseline intracellular concentration is so low that even a small influx
may increase it by orders of magnitude), but the permeabilities of the
ions can change in a fraction of a millisecond, as a result of
activation of ligand-gated ion channels. The change in membrane
potential can be either large or small, depending on how many ion
channels are activated and what type they are, and can be either long or
short, depending on the lengths of time that the channels remain open.
Changes of this type are referred to as graded potentials, in contrast to action potentials, which have a fixed amplitude and time course.
As can be derived from the Goldman equation
shown above, the effect of increasing the permeability of a membrane to
a particular type of ion shifts the membrane potential toward the
reversal potential for that ion. Thus, opening Na+ channels shifts the membrane potential toward the Na+ reversal potential, which is usually around +100 mV. Likewise, opening K+ channels shifts the membrane potential toward about –90 mV, and opening Cl− channels shifts it toward about –70 mV (resting potential of most membranes). Thus, Na+ channels shift the membrane potential in a positive direction, K+ channels shift it in a negative direction (except when the membrane is hyperpolarized to a value more negative than the K+ reversal potential), and Cl− channels tend to shift it towards the resting potential.
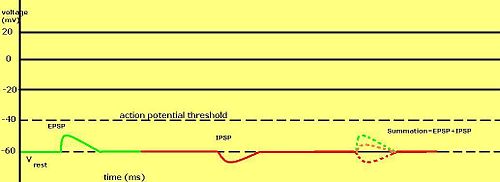
Graph displaying an EPSP, an IPSP, and the summation of an EPSP and an IPSP
Graded membrane potentials are particularly important in neurons, where they are produced by synapses—a
temporary change in membrane potential produced by activation of a
synapse by a single graded or action potential is called a postsynaptic potential. Neurotransmitters that act to open Na+ channels typically cause the membrane potential to become more positive, while neurotransmitters that activate K+ channels typically cause it to become more negative; those that inhibit these channels tend to have the opposite effect.
Whether a postsynaptic potential is considered excitatory or
inhibitory depends on the reversal potential for the ions of that
current, and the threshold for the cell to fire an action potential
(around –50mV). A postsynaptic current with a reversal potential above
threshold, such as a typical Na+ current, is considered excitatory. A current with a reversal potential below threshold, such as a typical K+
current, is considered inhibitory. A current with a reversal potential
above the resting potential, but below threshold, will not by itself
elicit action potentials, but will produce subthreshold membrane potential oscillations. Thus, neurotransmitters that act to open Na+ channels produce excitatory postsynaptic potentials, or EPSPs, whereas neurotransmitters that act to open K+ or Cl− channels typically produce inhibitory postsynaptic potentials,
or IPSPs. When multiple types of channels are open within the same time
period, their postsynaptic potentials summate (are added together).
Other values
From the viewpoint of biophysics, the resting
membrane potential is merely the membrane potential that results from
the membrane permeabilities that predominate when the cell is resting.
The above equation of weighted averages always applies, but the
following approach may be more easily visualized.
At any given moment, there are two factors for an ion that determine how
much influence that ion will have over the membrane potential of a
cell:
- That ion's driving force
- That ion's permeability
If the driving force is high, then the ion is being "pushed" across
the membrane. If the permeability is high, it will be easier for the
ion to diffuse across the membrane.
- Driving force is the net electrical force available to move that ion across the membrane. It is calculated as the difference between the voltage that the ion "wants" to be at (its equilibrium potential) and the actual membrane potential (Em). So, in formal terms, the driving force for an ion = Em - Eion
- For example, at our earlier calculated resting potential of −73 mV, the driving force on potassium is 7 mV : (−73 mV) − (−80 mV) = 7 mV. The driving force on sodium would be (−73 mV) − (60 mV) = −133 mV.
- Permeability is a measure of how easily an ion can cross the membrane. It is normally measured as the (electrical) conductance and the unit, siemens, corresponds to 1 C·s−1·V−1, that is one coulomb per second per volt of potential.
So, in a resting membrane, while the driving force for potassium is
low, its permeability is very high. Sodium has a huge driving force but
almost no resting permeability. In this case, potassium carries about
20 times more current than sodium, and thus has 20 times more influence
over Em than does sodium.
However, consider another case—the peak of the action potential.
Here, permeability to Na is high and K permeability is relatively low.
Thus, the membrane moves to near ENa and far from EK.
The more ions are permeant the more complicated it becomes to
predict the membrane potential. However, this can be done using the Goldman-Hodgkin-Katz equation
or the weighted means equation. By plugging in the concentration
gradients and the permeabilities of the ions at any instant in time, one
can determine the membrane potential at that moment. What the GHK
equations means is that, at any time, the value of the membrane
potential will be a weighted average of the equilibrium potentials of
all permeant ions. The "weighting" is the ions relative permeability
across the membrane.
Effects and implications
While
cells expend energy to transport ions and establish a transmembrane
potential, they use this potential in turn to transport other ions and
metabolites such as sugar. The transmembrane potential of the mitochondria drives the production of ATP, which is the common currency of biological energy.
Cells may draw on the energy they store in the resting potential
to drive action potentials or other forms of excitation. These changes
in the membrane potential enable communication with other cells (as with
action potentials) or initiate changes inside the cell, which happens
in an egg when it is fertilized by a sperm.
In neuronal cells, an action potential begins with a rush of
sodium ions into the cell through sodium channels, resulting in
depolarization, while recovery involves an outward rush of potassium
through potassium channels. Both of these fluxes occur by passive diffusion.



![E_{eq,K^+} = \frac{RT}{zF} \ln \frac{[K^+]_{o}}{[K^+]_{i}} ,](https://wikimedia.org/api/rest_v1/media/math/render/svg/b7eb1afcc5d7a5032ff24be5c403b43b1307b01e)