Electrophysiology (from Greek ἥλεκτρον, ēlektron, "amber" [see the etymology of "electron"]; φύσις, physis, "nature, origin"; and -λογία, -logia) is the branch of physiology that studies the electrical properties of biological cells and tissues. It involves measurements of voltage changes or electric current or manipulations on a wide variety of scales from single ion channel proteins to whole organs like the heart. In neuroscience, it includes measurements of the electrical activity of neurons, and, in particular, action potential activity. Recordings of large-scale electric signals from the nervous system, such as electroencephalography, may also be referred to as electrophysiological recordings. They are useful for electrodiagnosis and monitoring.
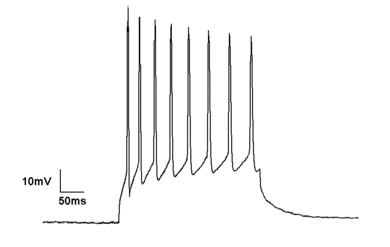
"Current
Clamp" is a common technique in electrophysiology. This is a whole-cell
current clamp recording of a neuron firing due to it being depolarized
by current injection
Definition and scope
Classical electrophysiological techniques
Principle and mechanisms
Electrophysiology is the branch of physiology that pertains broadly to the flow of ions (ion current)
in biological tissues and, in particular, to the electrical recording
techniques that enable the measurement of this flow. Classical
electrophysiology techniques involve placing electrodes into various preparations of biological tissue. The principal types of electrodes are:
- simple solid conductors, such as discs and needles (singles or arrays, often insulated except for the tip),
- tracings on printed circuit boards or flexible polymers, also insulated except for the tip, and
- hollow tubes filled with an electrolyte, such as glass pipettes filled with potassium chloride solution or another electrolyte solution.
The principal preparations include:
- living organisms,
- excised tissue (acute or cultured),
- dissociated cells from excised tissue (acute or cultured),
- artificially grown cells or tissues, or
- hybrids of the above.
Neuronal electrophysiology is the study of electrical properties of
biological cells and tissues within the nervous system. With neuronal
electrophysiology doctors and specialists can determine how neuronal
disorders happen, by looking at the individual's brain activity.
Activity such as which portions of the brain light up during any
situations encountered.
If an electrode is small enough (micrometers) in diameter, then the electrophysiologist may choose to insert the tip into a single cell. Such a configuration allows direct observation and recording of the intracellular
electrical activity of a single cell. However, this invasive setup
reduces the life of the cell and causes a leak of substances across the
cell membrane. Intracellular activity may also be observed using a
specially formed (hollow) glass pipette containing an electrolyte. In
this technique, the microscopic pipette tip is pressed against the cell
membrane, to which it tightly adheres by an interaction between glass
and lipids of the cell membrane. The electrolyte within the pipette may
be brought into fluid continuity with the cytoplasm by delivering a
pulse of negative pressure to the pipette in order to rupture the small
patch of membrane encircled by the pipette rim (whole-cell recording).
Alternatively, ionic continuity may be established by "perforating" the
patch by allowing exogenous pore-forming agent within the electrolyte
to insert themselves into the membrane patch (perforated patch recording). Finally, the patch may be left intact (patch recording).
The electrophysiologist may choose not to insert the tip into a
single cell. Instead, the electrode tip may be left in continuity with
the extracellular space. If the tip is small enough, such a
configuration may allow indirect observation and recording of action potentials from a single cell, termed single-unit recording.
Depending on the preparation and precise placement, an extracellular
configuration may pick up the activity of several nearby cells
simultaneously, termed multi-unit recording.
As electrode size increases, the resolving power decreases.
Larger electrodes are sensitive only to the net activity of many cells,
termed local field potentials.
Still larger electrodes, such as uninsulated needles and surface
electrodes used by clinical and surgical neurophysiologists, are
sensitive only to certain types of synchronous activity within
populations of cells numbering in the millions.
Other classical electrophysiological techniques include single channel recording and amperometry.
Electrographic modalities by body part
Electrophysiological recording in general is sometimes called electrography (from electro- + -graphy, "electrical recording"), with the record thus produced being an electrogram. However, the word electrography has other senses (including electrophotography), and the specific types of electrophysiological recording are usually called by specific names, constructed on the pattern of electro- + [body part combining form] + -graphy (abbreviation ExG). Relatedly, the word electrogram (not being needed for those other senses)
often carries the specific meaning of intracardiac electrogram, which
is like an electrocardiogram but with some invasive leads (inside the
heart) rather than only noninvasive leads (on the skin).
Electrophysiological recording for clinical diagnostic purposes is included within the category of electrodiagnostic testing. The various "ExG" modes are as follows:
| Modality | Abbreviation | Body part | Prevalence in clinical use |
|---|---|---|---|
| electrocardiography | ECG or EKG | heart (specifically, the cardiac muscle), with cutaneous electrodes (noninvasive) | 1—very common |
| electroatriography | EAG | atrial cardiac muscle | 3—uncommon |
| electroventriculography | EVG | ventricular cardiac muscle | 3—uncommon |
| intracardiac electrogram | EGM | heart (specifically, the cardiac muscle), with intracardiac electrodes (invasive) | 2—somewhat common |
| electroencephalography | EEG | brain (usually the cerebral cortex), with extracranial electrodes | 2—somewhat common |
| electrocorticography | ECoG or iEEG | brain (specifically the cerebral cortex), with intracranial electrodes | 2—somewhat common |
| electromyography | EMG | muscles throughout the body (usually skeletal, occasionally smooth) | 1—very common |
| electrooculography | EOG | eye—entire globe | 2—somewhat common |
| electroretinography | ERG | eye—retina specifically | 2—somewhat common |
| electronystagmography | ENG | eye—via the corneoretinal potential | 2—somewhat common |
| electroolfactography | EOG | olfactory epithelium in mammals | 3—uncommon |
| electroantennography | EAG | olfactory receptors in arthropod antennae | 4—not applicable clinically |
| electrocochleography | ECOG or ECochG | cochlea | 2—somewhat common |
| electrogastrography | EGG | stomach smooth muscle | 2—somewhat common |
| electrogastroenterography | EGEG | stomach and bowel smooth muscle | 2—somewhat common |
| electroglottography | EGG | glottis | 3—uncommon |
| electropalatography | EPG | palatal contact of tongue | 3—uncommon |
| electroarteriography | EAG | arterial flow via streaming potential detected through skin[2] | 3—uncommon |
| electroblepharography | EBG | eyelid muscle | 3—uncommon |
| electrodermography | EDG | skin | 3—uncommon |
| electrohysterography | EHG | uterus | 3—uncommon |
| electroneuronography | ENeG or ENoG | nerves | 3—uncommon |
| electropneumography | EPG | lungs (chest movements) | 3—uncommon |
| electrospinography | ESG | spinal cord | 3—uncommon |
| electrovomerography | EVG | vomeronasal organ | 3—uncommon |
Optical electrophysiological techniques
Optical
electrophysiological techniques were created by scientists and
engineers to overcome one of the main limitations of classical
techniques. Classical techniques allow observation of electrical
activity at approximately a single point within a volume of tissue.
Essentially, classical techniques singularize a distributed phenomenon.
Interest in the spatial distribution of bioelectric activity prompted
development of molecules capable of emitting light in response to their
electrical or chemical environment. Examples are voltage sensitive dyes and fluorescing proteins.
After introducing one or more such compounds into tissue via perfusion,
injection or gene expression, the 1 or 2-dimensional distribution of
electrical activity may be observed and recorded.
Intracellular recording
Intracellular recording
involves measuring voltage and/or current across the membrane of a
cell. To make an intracellular recording, the tip of a fine (sharp)
microelectrode must be inserted inside the cell, so that the membrane potential
can be measured. Typically, the resting membrane potential of a
healthy cell will be -60 to -80 mV, and during an action potential the
membrane potential might reach +40 mV.
In 1963, Alan Lloyd Hodgkin and Andrew Fielding Huxley
won the Nobel Prize in Physiology or Medicine for their contribution to
understanding the mechanisms underlying the generation of action
potentials in neurons. Their experiments involved intracellular
recordings from the giant axon
of Atlantic squid (Loligo pealei), and were among the first
applications of the "voltage clamp" technique.
Today, most microelectrodes used for intracellular recording are glass
micropipettes, with a tip diameter of < 1 micrometre, and a
resistance of several megohms. The micropipettes are filled with a
solution that has a similar ionic composition to the intracellular fluid
of the cell. A chlorided silver wire inserted into the pipet connects
the electrolyte electrically to the amplifier and signal processing
circuit. The voltage measured by the electrode is compared to the
voltage of a reference electrode, usually a silver chloride-coated
silver wire in contact with the extracellular fluid around the cell. In
general, the smaller the electrode tip, the higher its electrical resistance,
so an electrode is a compromise between size (small enough to penetrate
a single cell with minimum damage to the cell) and resistance (low
enough so that small neuronal signals can be discerned from thermal
noise in the electrode tip).
Voltage clamp
The
voltage clamp uses a negative feedback mechanism. The membrane
potential amplifier measures membrane voltage and sends output to the
feedback amplifier. The feedback amplifier subtracts the membrane
voltage from the command voltage, which it receives from the signal
generator. This signal is amplified and returned into the cell via the
recording electrode.
The voltage clamp technique allows an experimenter to "clamp" the cell potential at a chosen value. This makes it possible to measure how much ionic current crosses a cell's membrane at any given voltage. This is important because many of the ion channels in the membrane of a neuron are voltage-gated ion channels,
which open only when the membrane voltage is within a certain range.
Voltage clamp measurements of current are made possible by the
near-simultaneous digital subtraction of transient capacitive currents
that pass as the recording electrode and cell membrane are charged to
alter the cell's potential.
Current clamp
The current clamp technique records the membrane potential
by injecting current into a cell through the recording electrode.
Unlike in the voltage clamp mode, where the membrane potential is held
at a level determined by the experimenter, in "current clamp" mode the
membrane potential is free to vary, and the amplifier records whatever
voltage the cell generates on its own or as a result of stimulation.
This technique is used to study how a cell responds when electric
current enters a cell; this is important for instance for understanding
how neurons respond to neurotransmitters that act by opening membrane ion channels.
Most current-clamp amplifiers provide little or no amplification
of the voltage changes recorded from the cell. The "amplifier" is
actually an electrometer,
sometimes referred to as a "unity gain amplifier"; its main purpose is
to reduce the electrical load on the small signals (in the mV range)
produced by cells so that they can be accurately recorded by low-impedance
electronics. The amplifier increases the current behind the signal
while decreasing the resistance over which that current passes.
Consider this example based on Ohm's law: A voltage of 10 mV is
generated by passing 10 nanoamperes of current across 1 MΩ of resistance. The electrometer changes this "high impedance signal" to a "low impedance signal" by using a voltage follower circuit. A voltage follower reads the voltage on the input (caused by a small current through a big resistor).
It then instructs a parallel circuit that has a large current source
behind it (the electrical mains) and adjusts the resistance of that
parallel circuit to give the same output voltage, but across a lower
resistance.
Patch-clamp recording
The cell-attached patch clamp uses a micropipette attached to the cell membrane to allow recording from a single ion channel.
This technique was developed by Erwin Neher and Bert Sakmann who received the Nobel Prize in 1991.
Conventional intracellular recording involves impaling a cell with a
fine electrode; patch-clamp recording takes a different approach. A
patch-clamp microelectrode is a micropipette with a relatively large tip
diameter. The microelectrode is placed next to a cell, and gentle
suction is applied through the microelectrode to draw a piece of the
cell membrane (the 'patch') into the microelectrode tip; the glass tip
forms a high resistance 'seal' with the cell membrane. This
configuration is the "cell-attached" mode, and it can be used for
studying the activity of the ion channels that are present in the patch
of membrane.
If more suction is now applied, the small patch of membrane in the
electrode tip can be displaced, leaving the electrode sealed to the rest
of the cell. This "whole-cell" mode allows very stable intracellular
recording. A disadvantage (compared to conventional intracellular
recording with sharp electrodes) is that the intracellular fluid of the
cell mixes with the solution inside the recording electrode, and so some
important components of the intracellular fluid can be diluted. A
variant of this technique, the "perforated patch" technique, tries to
minimise these problems.
Instead of applying suction to displace the membrane patch from the
electrode tip, it is also possible to make small holes on the patch with
pore-forming agents so that large molecules such as proteins can stay
inside the cell and ions can pass through the holes freely. Also the
patch of membrane can be pulled away from the rest of the cell. This
approach enables the membrane properties of the patch to be analysed
pharmacologically.
Sharp electrode recording
In
situations where one wants to record the potential inside the cell
membrane with minimal effect on the ionic constitution of the
intracellular fluid a sharp electrode can be used. These micropipettes
(electrodes) are again like those for patch clamp pulled from glass
capillaries, but the pore is much smaller so that there is very little
ion exchange between the intracellular fluid and the electrolyte in the
pipette. The resistance of the micropipette electrode is tens or
hundreds of MΩ. Often the tip of the electrode is filled with various kinds of dyes like Lucifer yellow
to fill the cells recorded from, for later confirmation of their
morphology under a microscope. The dyes are injected by applying a
positive or negative, DC or pulsed voltage to the electrodes depending
on the polarity of the dye.
Extracellular recording
Single-unit recording
An electrode introduced into the brain of a living animal will detect
electrical activity that is generated by the neurons adjacent to the
electrode tip. If the electrode is a microelectrode, with a tip size of
about 1 micrometre, the electrode will usually detect the activity of at
most one neuron. Recording in this way is in general called
"single-unit" recording. The action potentials recorded are very much
like the action potentials that are recorded intracellularly, but the
signals are very much smaller (typically about 1 mV). Most recordings of
the activity of single neurons in anesthetized and conscious animals
are made in this way. Recordings of single neurons in living animals
have provided important insights into how the brain processes
information. For example, David Hubel and Torsten Wiesel recorded the activity of single neurons in the primary visual cortex of the anesthetized cat, and showed how single neurons in this area respond to very specific features of a visual stimulus. Hubel and Wiesel were awarded the Nobel Prize in Physiology or Medicine in 1981.
Multi-unit recording
If
the electrode tip is slightly larger, then the electrode might record
the activity generated by several neurons. This type of recording is
often called "multi-unit recording", and is often used in conscious
animals to record changes in the activity in a discrete brain area
during normal activity. Recordings from one or more such electrodes that
are closely spaced can be used to identify the number of cells around
it as well as which of the spikes come from which cell. This process is
called spike sorting
and is suitable in areas where there are identified types of cells with
well defined spike characteristics.
If the electrode tip is bigger still, in general the activity of
individual neurons cannot be distinguished but the electrode will still
be able to record a field potential generated by the activity of many
cells.
Field potentials
A schematic diagram showing a field potential recording from rat hippocampus. At the left is a schematic diagram of a presynaptic terminal
and postsynaptic neuron. This is meant to represent a large population
of synapses and neurons. When the synapse releases glutamate onto the
postsynaptic cell, it opens ionotropic glutamate receptor channels. The
net flow of current is inward, so a current sink is generated. A
nearby electrode (#2) detects this as a negativity. An intracellular electrode placed inside the cell body (#1) records the change in membrane potential that the incoming current causes.
Extracellular field potentials
are local current sinks or sources that are generated by the collective
activity of many cells. Usually, a field potential is generated by the
simultaneous activation of many neurons by synaptic transmission.
The diagram to the right shows hippocampal synaptic field potentials.
At the right, the lower trace shows a negative wave that corresponds to
a current sink caused by positive charges entering cells through
postsynaptic glutamate receptors,
while the upper trace shows a positive wave that is generated by the
current that leaves the cell (at the cell body) to complete the circuit.
For more information, see local field potential.
Amperometry
Amperometry
uses a carbon electrode to record changes in the chemical composition
of the oxidized components of a biological solution. Oxidation and
reduction is accomplished by changing the voltage at the active surface
of the recording electrode in a process known as "scanning". Because
certain brain chemicals lose or gain electrons at characteristic
voltages, individual species can be identified. Amperometry has been
used for studying exocytosis in the nervous and endocrine systems. Many
monoamine neurotransmitters; e.g., norepinephrine (noradrenalin), dopamine, and serotonin
(5-HT) are oxidizable. The method can also be used with cells that do
not secrete oxidizable neurotransmitters by "loading" them with 5-HT or
dopamine.
Planar patch clamp
Planar patch clamp is a novel method developed for high throughput electrophysiology. Instead of positioning a pipette on an adherent cell, cell suspension is pipetted on a chip containing a microstructured aperture.
A single cell is then positioned on the hole by suction and a tight connection (Gigaseal) is formed.
The planar geometry offers a variety of advantages compared to the classical experiment:
- It allows for integration of microfluidics, which enables automatic compound application for ion channel screening.
- The system is accessible for optical or scanning probe techniques.
- Perfusion of the intracellular side can be performed.
- Scanning electron microscope image of a planar patch clamp chip. Both the pipette and the chip are made from borosilicate glass.
Other methods
Solid-supported membrane (SSM)-based
With this electrophysiological approach, proteoliposomes, membrane vesicles,
or membrane fragments containing the channel or transporter of interest
are adsorbed to a lipid monolayer painted over a functionalized
electrode. This electrode consists of a glass support, a chromium layer, a gold layer, and an octadecyl mercaptane
monolayer. Because the painted membrane is supported by the electrode,
it is called a solid-supported membrane. It is important to note that
mechanical perturbations, which usually destroy a biological lipid
membrane, do not influence the life-time of an SSM. The capacitive
electrode (composed of the SSM and the absorbed vesicles) is so
mechanically stable that solutions may be rapidly exchanged at its
surface. This property allows the application of rapid substrate/ligand
concentration jumps to investigate the electrogenic activity of the
protein of interest, measured via capacitive coupling between the
vesicles and the electrode.
Bioelectric recognition assay (BERA)
The
bioelectric recognition assay (BERA) is a novel method for
determination of various chemical and biological molecules by measuring
changes in the membrane potential of cells immobilized in a gel matrix.
Apart from the increased stability of the electrode-cell interface,
immobilization preserves the viability and physiological functions of
the cells. BERA is used primarily in biosensor applications in order to assay analytes that can interact with the immobilized cells
by changing the cell membrane potential. In this way, when a positive
sample is added to the sensor, a characteristic, "signature-like" change
in electrical potential occurs. BERA is the core technology behind the
recently launched pan-European FOODSCAN project, about pesticide and
food risk assessment in Europe. BERA has been used for the detection of human viruses (hepatitis B and C viruses and herpes viruses), veterinary disease agents (foot and mouth disease virus, prions, and blue tongue virus), and plant viruses (tobacco and cucumber viruses)
in a specific, rapid (1–2 minutes), reproducible, and cost-efficient
fashion. The method has also been used for the detection of
environmental toxins, such as pesticides and mycotoxins in food, and 2,4,6-trichloroanisole in cork and wine, as well as the determination of very low concentrations of the superoxide anion in clinical samples.
A BERA sensor has two parts:
- The consumable biorecognition elements
- The electronic read-out device with embedded artificial intelligence.
A recent advance is the development of a technique called molecular
identification through membrane engineering (MIME). This technique
allows for building cells with defined specificity for virtually any
molecule of interest, by embedding thousands of artificial receptors
into the cell membrane.
Computational electrophysiology
While
not strictly constituting an experimental measurement, methods have
been developed to examine the conductive properties of proteins and
biomembranes in silico. These are mainly molecular dynamics simulations in which a model system like a lipid bilayer is subjected to an externally applied voltage. Studies using these setups have been able to study dynamical phenomena like electroporation of membranes and ion translocation by channels.
The benefit of such methods is the high level of detail of the
active conduction mechanism, given by the inherently high resolution and
data density that atomistic simulation affords. There are significant
drawbacks, given by the uncertainty of the legitimacy of the model and
the computational cost of modeling systems that are large enough and
over sufficient timescales to be considered reproducing the macroscopic
properties of the systems themselves. While atomistic simulations may
access timescales close to, or into the microsecond domain, this is
still several orders of magnitude lower than even the resolution of
experimental methods such as patch-clamping.
Clinical electrophysiology
Clinical electrophysiology is the study of how electrophysiological principles and technologies can be applied to human health. For example, clinical cardiac electrophysiology
is the study of the electrical properties which govern heart rhythm and
activity. Cardiac electrophysiology can be used to observe and treat
disorders such as arrhythmia
(irregular heartbeat). For example, a doctor may insert a catheter
containing an electrode into the heart to record the heart muscle's
electrical activity.
Another example of clinical electrophysiology is clinical neurophysiology. In this medical specialty, doctors measure the electrical properties of the brain, spinal cord, and nerves. Scientists such as Duchenne de Boulogne (1806–1875) and Nathaniel A. Buchwald (1924–2006) are considered to have greatly advanced the field of neurophysiology, enabling its clinical applications.
Clinical reporting guidelines
Minimum Information (MI) standards or reporting guidelines specify the minimum amount of meta data
(information) and data required to meet a specific aim or aims in a
clinical study. The "Minimum Information about a Neuroscience
investigation" (MINI) family of reporting guideline documents aims to
provide a consistent set of guidelines in order to report an
electrophysiology experiment. In practice a MINI module comprises a
checklist of information that should be provided (for example about the
protocols employed) when a data set is described for publication.