| Mycobacterium tuberculosis | |
|---|---|

| |
| M. tuberculosis bacterial colonies | |
| Scientific classification | |
| Domain: | |
| Phylum: | |
| Class: |
Actinobacteria
|
| Order: | |
| Family: | |
| Genus: | |
| Species: |
M. tuberculosis
|
| Binomial name | |
| Mycobacterium tuberculosis
Zopf 1883
| |
| Synonyms | |
|
Tubercle bacillus Koch 1882
| |
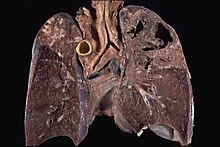
M. tuberculosis in the lungs
Mycobacterium tuberculosis (M. tb) is a species of pathogenic bacteria in the family Mycobacteriaceae and the causative agent of tuberculosis. First discovered in 1882 by Robert Koch, M. tuberculosis has an unusual, waxy coating on its cell surface primarily due to the presence of mycolic acid. This coating makes the cells impervious to Gram staining, and as a result, M. tuberculosis can appear either Gram-negative or Gram-positive. Acid-fast stains such as Ziehl-Neelsen, or fluorescent stains such as auramine are used instead to identify M. tuberculosis with a microscope. The physiology of M. tuberculosis is highly aerobic and requires high levels of oxygen. Primarily a pathogen of the mammalian respiratory system, it infects the lungs. The most frequently used diagnostic methods for tuberculosis are the tuberculin skin test, acid-fast stain, culture, and polymerase chain reaction.
Microbiology
M. tuberculosis is part of a complex that has at least 9 members: M. tuberculosis sensu stricto, M. africanum, M. canetti, M. bovis, M. caprae, M. microti, M. pinnipedii, M. mungi, and M. orygis. It requires oxygen to grow, does not produce spores, and is nonmotile. M. tuberculosis
divides every 15–20 hours. This is extremely slow compared with other
bacteria, which tend to have division times measured in minutes (Escherichia coli can divide roughly every 20 minutes). It is a small bacillus that can withstand weak disinfectants and can survive in a dry state for weeks. Its unusual cell wall is rich in lipids such as mycolic acid and is likely responsible for its resistance to desiccation and is a key virulence factor.
Microscopy
Other bacteria are commonly identified with a microscope by staining them with Gram stain. However, the mycolic acid in the cell wall of M. tuberculosis does not absorb the stain. Instead, acid-fast stains such as Ziehl-Neelsen stain, or fluorescent stains such as auramine are used.
Cells are curved rod-shaped and are often seen wrapped together, due to
the presence of fatty acids in the cell wall that stick together. This appearance is referred to as cording, like strands of cord that make up a rope. M. tuberculosis is characterized in tissue by caseating granulomas containing Langhans giant cells, which have a "horseshoe" pattern of nuclei.
Culture
M. tuberculosis can be grown in the laboratory. Compared to other commonly studied bacteria, M. tuberculosis has a remarkably slow growth rate, doubling roughly once per day. Commonly used media include liquids such as Middlebrook 7H9 or 7H12, egg-based solid media such as Lowenstein-Jensen, and solid agar-based such as Middlebrook 7H11 or 7H10.
Visible colonies require several weeks to grow on agar plates. It is
distinguished from other mycobacteria by its production of catalase and niacin. Other tests to confirm its identity include gene probes and MALDI-TOF.
Pathophysiology
Humans are the only known reservoirs of M. tuberculosis. A misconception is that M. tuberculosis
can be spread by shaking hands, making contact with toilet seats,
sharing food or drink, sharing toothbrushes, or kissing. It can only be
spread through air droplets originating from a person who has the disease either coughing, sneezing, speaking, or singing.
When in the lungs, M. tuberculosis is phagocytosed by alveolar macrophages, but they are unable to kill and digest the bacterium. Its cell wall prevents the fusion of the phagosome with the lysosome, which contains a host of antibacterial factors. Specifically, M. tuberculosis blocks the bridging molecule, early endosomal autoantigen 1 (EEA1);
however, this blockade does not prevent fusion of vesicles filled with
nutrients. Consequently, the bacteria multiply unchecked within the
macrophage. The bacteria also carry the UreC gene, which prevents acidification of the phagosome. In addition, production of the diterpene isotuberculosinol prevents maturation of the phagosome. The bacteria also evades macrophage-killing by neutralizing reactive nitrogen intermediates. More recently, it has been shown that M. tuberculosis secretes and covers itself in 1-tuberculosinyladenosine (1-TbAd), a special nucleoside that acts as an antacid, allowing it to neutralize pH and induce swelling in lysosomes. 1-TbAd is encoded by the gene Rv3378c.
It was also recently demonstrated that in M. tuberculosis
infections, PPM1A levels were upregulated, and this in turn would impact
the normal apoptotic response of macrophages to clear pathogens, as
PPM1A is involved in the intrinsic and extrinsic apoptotic pathways.
Hence, when PPM1A levels were increased, the expression of it would
inhibit the two apoptotic pathways.
With kinome analysis, the JNK/AP-1 signalling pathway was found to be a
downstream effector that PPM1A has a part to play in, and the apoptotic
pathway in macrophages are controlled in this manner. As a result of having apoptosis being suppressed, it provides M. tuberculosis with a safe replicative niche, and so the bacteria is able to maintain a latent state for a prolonged period of time.
Protective granulomas are formed due to the production of
cytokines and upregulation of proteins involved in recruitment.
Granulotomatous lesions are important in both regulating the immune
response and minimizing tissue damage. Moreover, T cells help maintain Mycobacterium within the granulomas.
The ability to construct M. tuberculosis mutants and test individual gene products for specific functions has significantly advanced the understanding of its pathogenesis and virulence factors. Many secreted and exported proteins are known to be important in pathogenesis. For example, one such virulence factor is cord factor (trehalose dimycolate), which serves to increase survival within its host. Resistant strains of M. tuberculosis
have developed resistance to more than one TB drug, due to mutations in
their genes. In addition, pre-existing first-line TB drugs such as
rifampicin and streptomycin have decreased efficiency in clearing
intracellular M. tuberculosis due not being able to effectively penetrate the macrophage niche.
JNK plays a key role in the control of apoptotic
pathways—intrinsic and extrinsic. In addition, it is also found to be a
substrate of PPM1A activity, hence the phosphorylation of JNK would cause apoptosis to occur. Since PPM1A levels are elevated during M. tuberculosis infections, by inhibiting the PPM1A signalling pathways, it could potentially be a therapeutic method to kill M. tuberculosis infected macrophages by restoring its normal apoptotic function in defence of pathogens. Therefore, by targeting the PPM1A-JNK signalling axis pathway, it could eliminate M. tuberculosis infected macrophages.
The ability to restore macrophage apoptosis to M. tuberculosis
infected ones could improve the current tuberculosis chemotherapy
treatment, as TB drugs can gain better access to the bacteria in the
niche. Therefore, decreasing the treatment time periods for M. tuberculosis infections.
Symptoms of M. tuberculosis include coughing that lasts for more than three weeks, hemoptysis, chest pain when breathing or coughing, weight loss, fatigue, fever, night sweats, chills, and loss of appetite. M. tuberculosis
also has the potential of spreading to other parts of the body. This
can cause blood in urine if the kidneys are affected, and back pain if
the spine is affected.
Strain variation
Typing
of strains is useful in the investigation of tuberculosis outbreaks,
because it gives the investigator evidence for or against transmission
from person to person. Consider the situation where person A has
tuberculosis and believes he acquired it from person B. If the bacteria
isolated from each person belong to different types, then transmission
from B to A is definitively disproved; however, if the bacteria are the
same strain, then this supports (but does not definitively prove) the
hypothesis that B infected A.
Until the early 2000s, M. tuberculosis strains were typed by pulsed field gel electrophoresis (PFGE). This has now been superseded by variable numbers of tandem repeats
(VNTR), which is technically easier to perform and allows better
discrimination between strains. This method makes use of the presence of
repeated DNA sequences within the M. tuberculosis genome.
Three generations of VNTR typing for M. tuberculosis are noted. The first scheme, called exact tandem repeat, used only five loci,
but the resolution afforded by these five loci was not as good as PFGE.
The second scheme, called mycobacterial interspersed repetitive unit,
had discrimination as good as PFGE.
The third generation (mycobacterial interspersed repetitive unit – 2)
added a further nine loci to bring the total to 24. This provides a
degree of resolution greater than PFGE and is currently the standard for
typing M. tuberculosis.
However, with regard to archaeological remains, additional evidence may
be required because of possible contamination from related soil
bacteria.
Antibiotic resistance in M. tuberculosis typically occurs
due to either the accumulation of mutations in the genes targeted by the
antibiotic or a change in titration of the drug. M. tuberculosis
is considered to be multidrug-resistant (MDR TB) if it has developed
drug resistance to both rifampicin and isoniazid, which are the most
important antibiotics used in treatment. Additionally, extensively
drug-resistant M. tuberculosis (XDR TB) is characterized by
resistance to both isoniazid and rifampin, plus any fluoroquinolone and
at least one of three injectable second-line drugs (i.e., amikacin,
kanamycin, or capreomycin).
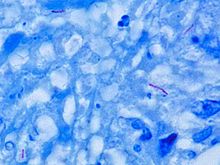
M. tuberculosis (stained red) in tissue (blue)
Cording M. tuberculosis (H37Rv strain) culture on the luminescent microscopy
Genome
The genome of the H37Rv strain was published in 1998.
Its size is 4 million base pairs, with 3,959 genes; 40% of these genes
have had their function characterized, with possible function postulated
for another 44%. Within the genome are also six pseudogenes.
The genome contains 250 genes involved in fatty acid metabolism, with 39 of these involved in the polyketide
metabolism generating the waxy coat. Such large numbers of conserved
genes show the evolutionary importance of the waxy coat to pathogen
survival. Furthermore, experimental studies have since validated the
importance of a lipid metabolism for M. tuberculosis, consisting
entirely of host-derived lipids such as fats and cholesterol. Bacteria
isolated from the lungs of infected mice were shown to preferentially
use fatty acids over carbohydrate substrates. M. tuberculosis can also grow on the lipid cholesterol
as a sole source of carbon, and genes involved in the cholesterol use
pathway(s) have been validated as important during various stages of the
infection lifecycle of M. tuberculosis, especially during the chronic phase of infection when other nutrients are likely not available.
About 10% of the coding capacity is taken up by the PE/PPE
gene families that encode acidic, glycine-rich proteins. These proteins
have a conserved N-terminal motif, deletion of which impairs growth in
macrophages and granulomas.
Nine noncoding sRNAs have been characterised in M. tuberculosis, with a further 56 predicted in a bioinformatics screen.
In 2013, a study on the genome of several sensitive, ultraresistant, and multiresistant M. tuberculosis
strains was made to study antibiotic resistance mechanisms. Results
reveal new relationships and drug resistance genes not previously
associated and suggest some genes and intergenic regions associated with
drug resistance may be involved in the resistance to more than one
drug. Noteworthy is the role of the intergenic regions in the
development of this resistance, and most of the genes proposed in this
study to be responsible for drug resistance have an essential role in
the development of M. tuberculosis.
Evolution
The M. tuberculosis complex evolved in Africa and most probably in the Horn of Africa. In addition to M. tuberculosis, the M. tuberculosis complex (MTBC) has a number of members infecting various animal species, these include M. africanum, M. bovis (Dassie's bacillus), M. caprae, M. microti, M. mungi, M. orygis, and M. pinnipedii. This group may also include the M. canettii
clade. These animal strains of MTBC do not strictly deserve species
status, as they are all closely related and embedded in the M. tuberculosis phylogeny, but for historic reasons, they currently hold species status.
The M. canettii clade – which includes M. prototuberculosis – is a group of smooth-colony Mycobacterium species. Unlike the established members of the M. tuberculosis
group, they undergo recombination with other species. The majority of
the known strains of this group have been isolated from the Horn of
Africa. The ancestor of M. tuberculosis appears to be M. canettii, first described in 1969.
The established members of the M. tuberculosis complex are
all clonal in their spread. The main human-infecting species have been
classified into seven lineages. Translating these lineages into the
terminology used for spoligotyping, a very crude genotyping methodology,
lineage 1 contains the East African-Indian (EAI), the Manila family of strains and some Manu (Indian) strains; lineage 2 is the Beijing group; lineage 3 includes the Central Asian (CAS) strains; lineage 4 includes the Ghana and Haarlem (H/T), Latin America-Mediterranean (LAM) and X strains; types 5 and 6 correspond to M. africanum and are observed predominantly and at high frequencies in West Africa. A seventh type has been isolated from the Horn of Africa. The other species of this complex belong to a number of spoligotypes and do not normally infect humans.
Lineages 2, 3 and 4 all share a unique deletion event (tbD1) and thus form a monophyletic group.
Types 5 and 6 are closely related to the animal strains of MTBC, which
do not normally infect humans. Lineage 3 has been divided into two
clades: CAS-Kili (found in Tanzania) and CAS-Delhi (found in India and Saudi Arabia).
Lineage 4 is also known as the Euro-American lineage. Subtypes
within this type include Latin American Mediterranean, Uganda I, Uganda
II, Haarlem, X, and Congo.
A much cited study reported that M. tuberculosis has co-evolved with human populations, and that the most recent common ancestor of the M. tuberculosis complex evolved between 40,000 and 70,000 years ago. However, a later study that included genome sequences from M. tuberculosis complex members extracted from three 1,000-year-old Peruvian mummies, came to quite different conclusions. If the most recent common ancestor of the M. tuberculosis
complex were 40,000 to 70,000 years old, this would necessitate an
evolutionary rate much lower than any estimates produced by genomic
analyses of heterochronous samples.
An analysis of over 3000 strains of M. bovis from 35 countries suggested an Africa origin for this species.
Co-evolution with modern humans
There are currently two narratives existing in parallel regarding the age of MTBC and how it has spread and co-evolved with humans through time. One study compared the M. tuberculosis
phylogeny to a human mitochondrial genome phylogeny and interpreted
these as being highly similar. Based on this, the study suggested that M. tuberculosis,
like humans, evolved in Africa and subsequently spread with
anatomically modern humans out of Africa across the world. By
calibrating the mutation rate of M. tuberculosis to match this
narrative, the study suggested that MTBC evolved 40,000 – 70,000 years
ago. Applying this time scale, the study found that the M. tuberculosis effective population size expanded during the Neolithic Demographic Transition (around 10,000 years ago) and suggested that M. tuberculosis
was able to adapt to changing human populations and that the historical
success of this pathogen was driven at least in part by dramatic
increases in human host population density. It has also been
demonstrated that after emigrating from one continent to another, a
human host's region of origin is predictive of which TB lineage they
carry, which could reflect either a stable association between host populations and specific M. tuberculosis lineages and/or social interactions that are shaped by shared cultural and geographic histories.
Regarding the congruence between human and M. tuberculosis phylogenies, a study relying on M. tuberculosis and human Y chromosome DNA sequences to formally assess the correlation between them, concluded that they are not congruent. Also, a more recent study which included genome sequences from M. tuberculosis complex members extracted from three 1,000-year-old Peruvian mummies, estimated that the most recent common ancestor of the M. tuberculosis complex lived only 4,000 – 6,000 years ago. The M. tuberculosis evolutionary rate estimated by the Bos et al. study is also supported by a study on Lineage 4 relying on genomic aDNA sequences from Hungarian mummies more than 200 years old.
In total, the evidence thus favors this more recent estimate of the age
of the MTBC most recent common ancestor, and thus that the global
evolution and dispersal of M. tuberculosis has occurred over the last 4,000–6,000 years.
Among the seven recognized lineages of M. tuberculosis, only two are truly global in their distribution: Lineages 2 and 4. Among these, Lineage 4 is most well dispersed, and is e.g.
almost totally dominating in the Americas. Lineage 4 was shown to have
evolved in or in the vicinity of Europe, and to have spread globally
with Europeans starting around the 13th century.
This study also found that Lineage 4 tuberculosis spread to the
Americas shortly after the European discovery of the continent in 1492,
and suggests that this represented the first introduction of human TB on
the continent (although animal strains have been found in human remains
predating Columbus. Similarly, Lineage 4 was found to have spread from Europe to Africa during the Age of Discovery, starting in the early 15th century. Similarly, Lineage 2 was introduced from Asia to Africa multiple times during the past 300 years. M. tuberculosis thus likely evolved in Africa
but the two globally distributed lineages evolved later; Lineage 2 in
East Asia and Lineage 4 in or around Europe, and has dispersed across
the globe from there.
It has been suggested that ancestral mycobacteria may have
infected early hominids in East Africa as early as three million years
ago.
Even though the MRCA of extant M. tuberculosis seems to have existed as
recently as 4,000-6,000 years ago, this does not necessarily suggest
that TB did not exist prior to that, it merely means that all M. tuberculosis strains circulating today can be traced back to a common ancestor that lived at that point in time.
Antibiotic resistance (ABR)
M. tuberculosis is a clonal organism and does not exchange DNA via horizontal gene transfer.
This, possibly combined with a relatively low rate of evolution, might
explain why the evolution of resistance has been relatively slow in the
species compared to some other major bacterial pathogens.
However, ABR is a very serious and growing challenge. Worst hit are
countries in the former Soviet republics, where ABR evolved and spread
at explosive levels following the fall of the Soviet Union. An extreme
example is Belarus, where a third of all new cases of tuberculosis are
multidrug-resistant.
Multidrug-resistant tuberculosis requires prolonged treatment with
expensive and often toxic drugs, and treatment failure is common.
Multidrug-resistant Tuberculosis (MDR-TB) is caused by an
organism that is resistant to at least isoniazid and rifampin, the two
most powerful TB drugs. These medications are utilized to treat all
people with TB illness. The majority of people with TB are cured by a
strictly followed, half-year medicate routine that is provided to
patients with support and supervision. Inappropriate or incorrect use of
antimicrobial medications, or utilization of ineffectual plans of
medications and untimely treatment interruption can cause drug
resistance, which can then be transmitted, especially in crowded
settings such as prisons and hospitals. In 2016, an estimated 490,000
people worldwide developed MDR-TB, and an additional 110,000 people with
rifampicin-resistant TB were also newly eligible for MDR-TB treatment.
The countries with the largest numbers of MDR-TB cases (47% of the
global total) were China, India and the Russian Federation.
Host genetics
The nature of the host-pathogen interaction between humans and M. tuberculosis
is considered to have a genetic component. A group of rare disorders
called Mendelian susceptibility to mycobacterial diseases was observed
in a subset of individuals with a genetic defect that results in
increased susceptibility to mycobacterial infection.
Early case and twin studies have indicated that genetic components are important in host susceptibility to M. tuberculosis. Recent genome-wide association studies (GWAS) have identified three genetic risk loci, including at positions 11p13 and 18q11. As is common in GWAS, the variants discovered have moderate effect sizes.
DNA repair
As an intracellular pathogen, M. tuberculosis
is exposed to a variety of DNA-damaging assaults, primarily from
host-generated antimicrobial toxic radicals. Exposure to reactive
oxygen species and/or reactive nitrogen species causes different types
of DNA damage including oxidation, depurination, methylation, and
deamination that can give rise to single- and double-strand breaks
(DSBs).
DnaE2 polymerase is upregulated in M. tuberculosis by several DNA-damaging agents, as well as during infection of mice. Loss of this DNA polymerase reduces the virulence of M. tuberculosis in mice. DnaE2 is an error-prone repair DNA repair polymerase that appears to contribute to M. tuberculosis survival during infection.
The two major pathways employed in repair of DSBs are homologous recombinational repair (HR) and nonhomologous end joining (NHEJ). Macrophage-internalized M. tuberculosis is able to persist if either of these pathways is defective, but is attenuated when both pathways are defective. This indicates that intracellular exposure of M. tuberculosis to reactive oxygen and/or reactive nitrogen species results in the formation of DSBs that are repaired by HR or NHEJ. However deficiency of DSB repair does not appear to impair M. tuberculosis virulence in animal models.
History
M. tuberculosis, then known as the "tubercle bacillus", was first described on 24 March 1882 by Robert Koch, who subsequently received the Nobel Prize in Physiology or Medicine for this discovery in 1905; the bacterium is also known as "Koch's bacillus".
M. tuberculosis has existed throughout history, but the
name has changed frequently over time. In 1720, though, the history of
tuberculosis started to take shape into what is known of it today; as
the physician Benjamin Marten described in his A Theory of Consumption, tuberculosis may be caused by small living creatures transmitted through the air to other patients.
This airborne disease is the deadliest infectious disease worldwide,
affecting nearly 2 billion people throughout the world currently. M. tuberculosis
has been proven to be present in women, children, and individuals with
viruses such as HIV or AIDS. It is easily passed through the air by
sneezing, coughing, or simply talking. A contaminated droplet can infect
any persons and they can become contaminated with M. tuberculosis. Thus, they become a part of the 1.8 billion people worldwide who are currently struggling with this disease.
Vaccine
The BCG vaccine (bacille Calmette-Guerin), which was derived from M. bovis, has had limited success in preventing tuberculosis. This vaccine is used in countries that are notorious for having cases of M. tuberculosis,
therefore it is not a recommended vaccine in the United States due to
the low risk of infection. To receive this vaccine, the individual is
required to go through a consultation process with an expert in M. tb
and is only given to those who meet the specific criteria.