Ketamine, one of the most common NMDA receptor antagonists.
NMDA receptor antagonists are a class of drugs that work to antagonize, or inhibit the action of, the N-Methyl-D-aspartate receptor (NMDAR). They are commonly used as anesthetics for animals and humans; the state of anesthesia they induce is referred to as dissociative anesthesia.
Several synthetic opioids function additionally as NMDAR-antagonists, such as pethidine, levorphanol, methadone, dextropropoxyphene, tramadol and ketobemidone.
Some NMDA receptor antagonists, such as ketamine, dextromethorphan (DXM), phencyclidine (PCP), methoxetamine (MXE), and nitrous oxide (N2O), are recreational drugs used for their dissociative, hallucinogenic, and euphoriant properties. When used recreationally, they are classified as dissociative drugs.
Uses and effects
NMDA receptor antagonists induce a state called dissociative anesthesia, marked by catalepsy, amnesia, and analgesia.
Ketamine is a favored anesthetic for emergency patients with unknown
medical history and in the treatment of burn victims because it
depresses breathing and circulation less than other anesthetics. Dextrorphan, a metabolite of dextromethorphan (one of the most commonly used cough suppressants in the world), is known to be an NMDA receptor antagonist.
Depressed NMDA receptor function is associated with an array of
negative symptoms. For example, NMDA receptor hypofunction that occurs
as the brain ages may be partially responsible for memory deficits associated with aging. Schizophrenia may also have to do with irregular NMDA receptor function (the glutamate hypothesis of schizophrenia). Increased levels of another NMDA antagonist, kynurenic acid, may aggravate the symptoms of schizophrenia, according to the "kynurenic hypothesis". NMDA receptor antagonists can mimic these problems; they sometimes induce "psychotomimetic" side effects, symptoms resembling psychosis. Such side effects caused by NMDA receptor inhibitors include hallucinations, paranoid delusions, confusion, difficulty concentrating, agitation, alterations in mood, nightmares, catatonia, ataxia, anesthesia, and learning and memory deficits.
Because of these psychotomimetic effects, NMDA receptor antagonists, especially phencyclidine, ketamine, and dextromethorphan,
are used as recreational drugs. At subanesthetic doses, these drugs
have mild stimulant effects and, at higher doses, begin inducing
dissociation and hallucinations, though these effects and the strength
thereof vary from drug to drug.
Most NMDA receptor antagonists are metabolized in the liver. Frequent administration of most NMDA receptor antagonists can lead to tolerance, whereby the liver will more quickly eliminate NMDA receptor antagonists from the bloodstream.
NMDA receptor antagonists are also under investigation as antidepressants.
Neurotoxicity
Although NMDA antagonists were once thought to reliably cause
neurotoxicity in humans in the form of Olney's lesions, recent research
suggests otherwise. Olney's lesions involve mass vacuolization of neurons observed in rodents.
However, many suggest that this is not a valid model of human use, and
studies conducted on primates have shown that use must be heavy and
chronic to cause neurotoxicity. A 2009 review found no evidence of ketamine-induced neuron death in humans.
However, temporary and permanent cognitive impairments have been shown
to occur in long-term or heavy human users of the NMDA antagonists PCP
and ketamine. A large-scale, longitudinal study found that current
frequent ketamine users have modest cognitive deficits, while infrequent
or former heavy users do not.
Many drugs have been found that lessen the risk of neurotoxicity from NMDA receptor antagonists. Centrally acting alpha 2 agonists such as clonidine and guanfacine are thought to most directly target the etiology
of NMDA neurotoxicity. Other drugs acting on various neurotransmitter
systems known to inhibit NMDA antagonist neurotoxicity include: anticholinergics, diazepam, barbiturates, ethanol, 5-HT2A serotonin receptor agonists, anticonvulsants, and muscimol.
Potential for treatment of excess excitotoxicity
Since NMDA receptor overactivation is implicated in excitotoxicity,
NMDA receptor antagonists have held much promise for the treatment of
conditions that involve excitotoxicity, including benzodiazepine
withdrawal, traumatic brain injury, stroke, and neurodegenerative diseases such as Alzheimer's, Parkinson's, and Huntington's. This is counterbalanced by the risk of developing Olney's lesions, which have only ever been observed in rodents, and studies have started to find agents that prevent this neurotoxicity.
Most clinical trials involving NMDA receptor antagonists have failed
due to unwanted side effects of the drugs; since the receptors also play
an important role in normal glutamatergic neurotransmission, blocking them causes side-effects. These results have not yet been reproduced in humans, however. Mild NMDA receptor antagonists like amitriptyline have been found to be helpful in benzodiazepine withdrawal.
Mechanism of action
Simplified model of NMDAR activation and various types of NMDAR blockers.
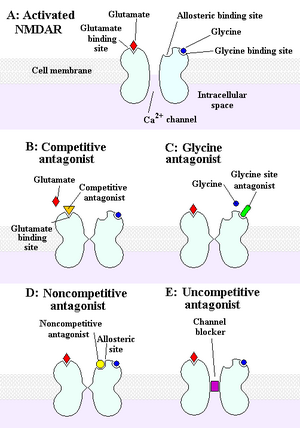
The NMDA receptor is an ionotropic receptor
that allows for the transfer of electrical signals between neurons in
the brain and in the spinal column. For electrical signals to pass, the
NMDA receptor must be open. To remain open, glutamate and glycine
must bind to the NMDA receptor. An NMDA receptor that has glycine and
glutamate bound to it and has an open ion channel is called "activated."
Chemicals that deactivate the NMDA receptor are called antagonists. NMDAR antagonists fall into four categories: Competitive antagonists, which bind to and block the binding site of the neurotransmitter
glutamate; glycine antagonists, which bind to and block the glycine
site; noncompetitive antagonists, which inhibit NMDARs by binding to allosteric sites; and uncompetitive antagonists, which block the ion channel by binding to a site within it.
Examples
Competitive antagonists
- AP5 (APV, R-2-amino-5-phosphonopentanoate)
- AP7 (2-amino-7-phosphonoheptanoic acid)
- CPPene (3-[(R)-2-carboxypiperazin-4-yl]-prop-2-enyl-1-phosphonic acid)
- Selfotel: an anxiolytic, anticonvulsant but with possible neurotoxic effects.
- Aspartame: artificial sweetener shown to have competitive NMDA inhibition.
Uncompetitive channel blockers
- 3-MeO-PCP: an analogue of PCP, but moderately more euphoric because its an SSRI.
- 8A-PDHQ: a high affinity PCP structural analogue.
- Amantadine: used for treating Parkinson's disease and influenza and Alzheimer's.
- Atomoxetine: a norepinephrine reuptake inhibitor used in the treatment of ADHD.
- AZD6765
- Agmatine: Blocks NMDA receptors and other cation ligand-gated channels. Can also potentiate opioid analgesia.
- Chloroform: an early anesthetic.
- Delucemine: also an SSRI with neuroprotective properties.
- Dextrallorphan: a more potent analogue of dextromethorphan.
- Dextromethorphan: a common antitussive found in cough medicines.
- Dextrorphan: active metabolite of dextromethorphan.
- Diphenidine: a novel designer drug sold on the internet.
- Dizocilpine (MK-801): an experimental drug used in scientific research.
- Ethanol: also known as alcohol, a widely used intoxicant.
- Eticyclidine: a slightly more potent dissociative anesthetic than phencyclidine but with greater nausea/unpleasant taste, that was discontinued early in its development due to these digestive complaints.
- Gacyclidine: an experimental drug developed for neuroprotection.
- Ketamine: a dissociative psychedelic with antidepressant properties used as an anesthesia in humans and animals, a possible treatment in bipolar disorder patients with Treatment-resistant depression, and used recreationally for its effects on the CNS
- Magnesium
- Memantine: treatment for Alzheimer's disease.
- Methoxetamine: a novel designer drug sold on the internet.
- Minocycline
- Nitromemantine: a novel memantine derivative.
- Nitrous oxide: used for anesthesia, particularly in dentistry.
- PD-137889: Potent NMDA receptor antagonist with roughly 30 times the potency of ketamine. Substitutes for PCP in animal studies.
- Phencyclidine: a dissociative anesthetic previously used in medicine, but its development was discontinued in the 1960s in favor of its successor ketamine due to its relatively high incidence of psychotomimetic effects. Abused recreational and legally controlled in most countries.
- Rolicyclidine: a less potent analogue of phencyclidine, but seems to be seldom, if ever, abused.
- Tenocyclidine: an analogue of phencyclidine that is more potent.
- Methoxydine: 4-meo-pcp
- Tiletamine: an animal anesthetic.
- Neramexane: a memantine analogue with nootropic, antidepressant properties. Also a nicotinic acetylcholine antagonist.
- Eliprodil: an anticonvulsant drug with neuroprotective properties.
- Etoxadrol: a potent dissociative similar to PCP.
- Dexoxadrol: similar to etoxadrol.
- WMS-2539: potent fluorinated derivative of dexoxadrol.
- NEFA: a moderate affinity antagonist.
- Remacemide: a low affinity antagonist also a sodium-channel blocker.
Non-competitive antagonists
- Aptiganel (Cerestat, CNS-1102): binds the Mg2+ binding site within the channel of the NMDA receptor.
- HU-211: an enantiomer of the potent cannabinoid HU-210 which lacks cannabinoid effects and instead acts as a potent non-competitive NMDA antagonist.
- Huperzine A
- Ibogaine: a naturally-occurring alkaloid found in plants of the family Apocynaceae. Has been used, albeit with limited evidence, to treat opioid and other addictions.
- Remacemide: principle metabolite is an uncompetitive antagonist with a low affinity for the binding site.
- Rhynchophylline an alkaloid, found in Kratom and Rubiaceae.
- Gabapentin: a calcium a2-d ligand that is commonly used in diabetic neuropathy.
Glycine antagonists
These drugs act at the glycine binding site:
- Rapastinel (GLYX-13) (weak partial agonist; IA = ~20%)
- NRX-1074 (weak partial agonist)
- 7-Chlorokynurenic acid
- 4-Chlorokynurenine (AV-101) (prodrug for 7-chlorokynurenic acid)
- 5,7-Dichlorokynurenic acid
- Kynurenic acid (a naturally occurring antagonist)
- TK-40 (competitive antagonist at the GluN1 glycine binding site)
- 1-Aminocyclopropanecarboxylic acid (ACPC)
- L-Phenylalanine, a naturally occurring amino acid (equilibrium dissociation constant (KB) from Schild regression is 573 μM).
- Xenon: an anesthetic.
Potencies
Uncompetitive channel blockers
| Compound | IC50 (nM) | Ki (nM) |
|---|---|---|
| (+)-MK-801 | 4.1 | 2.5 |
| Chlorophenidine | 14.6 | 9.3 |
| Diphenidine | 28.6 | 18.2 |
| Methoxyphenidine | 56.5 | 36.0 |
| Phencyclidine | 91 | 57.9 |
| Ketamine | 508.5 | 323.9 |
| Memantine | 594.2 | 378.4 |