| Coagulation | |
|---|---|
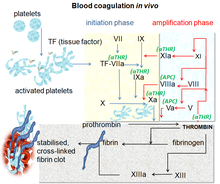
Blood coagulation pathways in vivo showing the central role played by thrombin
| |
| Health | Beneficial |
Coagulation, also known as clotting, is the process by which blood changes from a liquid to a gel, forming a blood clot. It potentially results in hemostasis, the cessation of blood loss from a damaged vessel, followed by repair. The mechanism of coagulation involves activation, adhesion and aggregation of platelets, as well as deposition and maturation of.
Coagulation begins almost instantly after an injury to the blood vessel has damaged the endothelium lining the blood vessel. Exposure of blood to the subendothelial space initiates two processes: changes in platelets, and the exposure of subendothelial tissue factor to plasma factor VII, which ultimately leads to cross-linked fibrin formation. Platelets immediately form a plug at the site of injury; this is called primary hemostasis. Secondary hemostasis occurs simultaneously: additional coagulation (clotting) factors beyond factor VII respond in a cascade to form fibrin strands, which strengthen the platelet plug.
Disorders of coagulation are disease states which can result in hemorrhage, bruising, or thrombosis.
Coagulation is highly conserved throughout biology. In all mammals, coagulation involves both a cellular (platelet) and a protein (coagulation factor) component. The system in humans has been the most extensively researched and is the best understood.
Physiology
The
interaction of vWF and GP1b alpha. The GP1b receptor on the surface of
platelets allows the platelet to bind to vWF, which is exposed upon
damage to vasculature. The vWF A1 domain (yellow) interacts with the
extracellular domain of GP1ba (blue).
Platelet activation
When
the endothelium is damaged, the normally isolated, underlying collagen
is exposed to circulating platelets, which bind directly to collagen
with collagen-specific glycoprotein Ia/IIa surface receptors. This adhesion is strengthened further by von Willebrand factor (vWF), which is released from the endothelium and from platelets; vWF forms additional links between the platelets' glycoprotein Ib/IX/V and A1 domain. This localization of platelets to the extracellular matrix promotes collagen interaction with platelet glycoprotein VI. Binding of collagen to glycoprotein VI
triggers a signaling cascade that results in activation of platelet
integrins. Activated integrins mediate tight binding of platelets to the
extracellular matrix. This process adheres platelets to the site of
injury.
Activated platelets release the contents of stored granules into the blood plasma. The granules include ADP, serotonin, platelet-activating factor (PAF), vWF, platelet factor 4, and thromboxane A2 (TXA2), which, in turn, activate additional platelets. The granules' contents activate a Gq-linked protein receptor cascade, resulting in increased calcium concentration in the platelets' cytosol. The calcium activates protein kinase C, which, in turn, activates phospholipase A2 (PLA2). PLA2 then modifies the integrin membrane glycoprotein IIb/IIIa, increasing its affinity to bind fibrinogen. The activated platelets change shape from spherical to stellate, and the fibrinogen cross-links with glycoprotein IIb/IIIa aid in aggregation of adjacent platelets (completing primary hemostasis).
Coagulation cascade
The classical blood coagulation pathway
Modern
coagulation pathway. Hand-drawn composite from similar drawings
presented by Professor Dzung Le, MD, PhD, at UCSD Clinical Chemistry
conferences on 14 and 21 October 2014. Original schema from Introduction
to Hematology by Samuel I. Rapaport. 2nd edition;Lippencott:1987. Dr Le
added the factor XI portion based on a paper from about year 2000. Dr.
Le's similar drawings presented the development of this cascade over 6
frames, like a comic.
The coagulation cascade of secondary hemostasis has two initial pathways which lead to fibrin formation. These are the contact activation pathway (also known as the intrinsic pathway), and the tissue factor pathway
(also known as the extrinsic pathway), which both lead to the same
fundamental reactions that produce fibrin. It was previously thought
that the two pathways of coagulation cascade were of equal importance,
but it is now known that the primary pathway for the initiation of blood
coagulation is the tissue factor (extrinsic) pathway. The pathways are a series of reactions, in which a zymogen (inactive enzyme precursor) of a serine protease and its glycoprotein
co-factor are activated to become active components that then catalyze
the next reaction in the cascade, ultimately resulting in cross-linked
fibrin. Coagulation factors are generally indicated by Roman numerals, with a lowercase a appended to indicate an active form.
The coagulation factors are generally serine proteases (enzymes), which act by cleaving downstream proteins. The exceptions are tissue factor, FV, FVIII, FXIII. Tissue factor, FV and FVIII are glycoproteins, and Factor XIII is a transglutaminase. The coagulation factors circulate as inactive zymogens.
The coagulation cascade is therefore classically divided into three pathways. The tissue factor and contact activation pathways both activate the "final common pathway" of factor X, thrombin and fibrin.
Tissue factor pathway (extrinsic)
The main role of the tissue factor pathway is to generate a "thrombin burst", a process by which thrombin,
the most important constituent of the coagulation cascade in terms of
its feedback activation roles, is released very rapidly. FVIIa
circulates in a higher amount than any other activated coagulation
factor. The process includes the following steps:
- Following damage to the blood vessel, FVII leaves the circulation and comes into contact with tissue factor (TF) expressed on tissue-factor-bearing cells (stromal fibroblasts and leukocytes), forming an activated complex (TF-FVIIa).
- TF-FVIIa activates FIX and FX.
- FVII is itself activated by thrombin, FXIa, FXII and FXa.
- The activation of FX (to form FXa) by TF-FVIIa is almost immediately inhibited by tissue factor pathway inhibitor (TFPI).
- FXa and its co-factor FVa form the prothrombinase complex, which activates prothrombin to thrombin.
- Thrombin then activates other components of the coagulation cascade, including FV and FVIII (which forms a complex with FIX), and activates and releases FVIII from being bound to vWF.
- FVIIIa is the co-factor of FIXa, and together they form the "tenase" complex, which activates FX; and so the cycle continues. ("Tenase" is a contraction of "ten" and the suffix "-ase" used for enzymes.)
Contact activation pathway (intrinsic)
The contact activation pathway begins with formation of the primary complex on collagen by high-molecular-weight kininogen (HMWK), prekallikrein, and FXII (Hageman factor). Prekallikrein is converted to kallikrein and FXII becomes FXIIa. FXIIa converts FXI into FXIa. Factor XIa activates FIX, which with its co-factor FVIIIa form the tenase complex, which activates FX to FXa. The minor role that the contact activation pathway has in initiating clot formation can be illustrated by the fact that patients with severe deficiencies of FXII, HMWK, and prekallikrein do not have a bleeding disorder. Instead, contact activation system seems to be more involved in inflammation, and innate immunity. Despite this, interference with the pathway may confer protection against thrombosis without a significant bleeding risk.
Final common pathway
The
division of coagulation in two pathways is arbitrary, originating from
laboratory tests in which clotting times were measured either after the
clotting was initiated by glass, the intrinsic pathway; or clotting was
initiated by thromboplastin (a mix of tissue factor and phospholipids),
the extrinsic pathway.
Further, the final common pathway scheme implies that prothrombin
is converted to thrombin only when acted upon by the intrinsic or
extrinsic pathways, which is an oversimplification. In fact, thrombin
is generated by activated platelets at the initiation of the platelet
plug, which in turn promotes more platelet activation.
Thrombin functions not only to convert fibrinogen to fibrin, it also activates Factors VIII and V and their inhibitor protein C (in the presence of thrombomodulin); and it activates Factor XIII, which forms covalent bonds that crosslink the fibrin polymers that form from activated monomers.
The coagulation cascade is maintained in a prothrombotic state by the continued activation of FVIII and FIX to form the tenase complex until it is down-regulated by the anticoagulant pathways.
Cell-based scheme of coagulation
A
newer model of coagulation mechanism explains the intricate combination
of cellular and biochemical events that occur during the coagulation
process in vivo.
Along with the procoagulant and anticoagulant plasma proteins, normal
physiologic coagulation requires the presence of two cell types for
formation of coagulation complexes: cells that express tissue factor
(usually extravascular) and platelets.
The coagulation process occurs in two phases. First is the
initiation phase, which occurs in tissue-factor-expressing cells. This
is followed by the propagation phase, which occurs on activated
platelets. The initiation phase, mediated by the tissue factor exposure,
proceeds via the classic extrinsic pathway and contributes to about 5%
of thrombin production. The amplified production of thrombin occurs via
the classic intrinsic pathway in the propagation phase; about 95% of
thrombin generated will be during this second phase.
Cofactors
Various substances are required for the proper functioning of the coagulation cascade:
Calcium and phospholipid
Calcium and phospholipid (a platelet
membrane constituent) are required for the tenase and prothrombinase
complexes to function. Calcium mediates the binding of the complexes via
the terminal gamma-carboxy residues on FXa and FIXa to the phospholipid
surfaces expressed by platelets, as well as procoagulant microparticles
or microvesicles shed from them. Calcium is also required at other points in the coagulation cascade.
Vitamin K
Vitamin K is an essential factor to a hepatic gamma-glutamyl carboxylase that adds a carboxyl group to glutamic acid residues on factors II, VII, IX and X, as well as Protein S, Protein C and Protein Z.
In adding the gamma-carboxyl group to glutamate residues on the
immature clotting factors, Vitamin K is itself oxidized. Another enzyme,
Vitamin K epoxide reductase
(VKORC), reduces vitamin K back to its active form. Vitamin K epoxide
reductase is pharmacologically important as a target of anticoagulant
drugs warfarin and related coumarins such as acenocoumarol, phenprocoumon, and dicumarol.
These drugs create a deficiency of reduced vitamin K by blocking VKORC,
thereby inhibiting maturation of clotting factors. Vitamin K deficiency
from other causes (e.g., in malabsorption) or impaired vitamin K metabolism in disease (e.g., in liver failure)
lead to the formation of PIVKAs (proteins formed in vitamin K absence),
which are partially or totally non-gamma carboxylated, affecting the
coagulation factors' ability to bind to phospholipid.
Regulators
Coagulation with arrows for negative and positive feedback.
Five mechanisms keep platelet activation and the coagulation cascade
in check. Abnormalities can lead to an increased tendency toward
thrombosis:
Protein C
Protein C is a major physiological anticoagulant. It is a vitamin K-dependent serine protease enzyme
that is activated by thrombin into activated protein C (APC). Protein C
is activated in a sequence that starts with Protein C and thrombin
binding to a cell surface protein thrombomodulin. Thrombomodulin binds these proteins in such a way that it activates
Protein C. The activated form, along with protein S and a phospholipid
as cofactors, degrades FVa and FVIIIa. Quantitative or qualitative
deficiency of either (protein C or protein S) may lead to thrombophilia (a tendency to develop thrombosis). Impaired action of Protein C (activated Protein C resistance), for example by having the "Leiden" variant of Factor V or high levels of FVIII, also may lead to a thrombotic tendency.
Antithrombin
Antithrombin is a serine protease inhibitor (serpin)
that degrades the serine proteases: thrombin, FIXa, FXa, FXIa, and
FXIIa. It is constantly active, but its adhesion to these factors is
increased by the presence of heparan sulfate (a glycosaminoglycan) or the administration of heparins
(different heparinoids increase affinity to FXa, thrombin, or both).
Quantitative or qualitative deficiency of antithrombin (inborn or
acquired, e.g., in proteinuria) leads to thrombophilia.
Tissue factor pathway inhibitor (TFPI)
Tissue factor pathway inhibitor (TFPI) limits the action of tissue factor (TF). It also inhibits excessive TF-mediated activation of FVII and FX.
Plasmin
Plasmin is generated by proteolytic cleavage of plasminogen, a plasma protein synthesized in the liver. This cleavage is catalyzed by tissue plasminogen activator
(t-PA), which is synthesized and secreted by endothelium. Plasmin
proteolytically cleaves fibrin into fibrin degradation products that
inhibit excessive fibrin formation.
Prostacyclin
Prostacyclin (PGI2) is released by endothelium and activates platelet Gs protein-linked receptors. This, in turn, activates adenylyl cyclase,
which synthesizes cAMP. cAMP inhibits platelet activation by decreasing
cytosolic levels of calcium and, by doing so, inhibits the release of
granules that would lead to activation of additional platelets and the
coagulation cascade.
Fibrinolysis
Eventually, blood clots are reorganised and resorbed by a process termed fibrinolysis. The main enzyme responsible for this process (plasmin) is regulated by various activators and inhibitors.
Role in immune system
The coagulation system overlaps with the immune system.
Coagulation can physically trap invading microbes in blood clots. Also,
some products of the coagulation system can contribute to the innate immune system by their ability to increase vascular permeability and act as chemotactic agents for phagocytic cells. In addition, some of the products of the coagulation system are directly antimicrobial. For example, beta-lysine, an amino acid produced by platelets during coagulation, can cause lysis of many Gram-positive bacteria by acting as a cationic detergent. Many acute-phase proteins of inflammation
are involved in the coagulation system. In addition, pathogenic
bacteria may secrete agents that alter the coagulation system, e.g. coagulase and streptokinase.
Assessment
Numerous tests are used to assess the function of the coagulation system:
- Common: aPTT, PT (also used to determine INR), fibrinogen testing (often by the Clauss method), platelet count, platelet function testing (often by PFA-100), thrombodynamics test.
- Other: TCT, bleeding time, mixing test (whether an abnormality corrects if the patient's plasma is mixed with normal plasma), coagulation factor assays, antiphospholipid antibodies, D-dimer, genetic tests (e.g. factor V Leiden, prothrombin mutation G20210A), dilute Russell's viper venom time (dRVVT), miscellaneous platelet function tests, thromboelastography (TEG or Sonoclot), euglobulin lysis time (ELT).
The contact activation (intrinsic) pathway is initiated by activation
of the "contact factors" of plasma, and can be measured by the activated partial thromboplastin time (aPTT) test.
The tissue factor (extrinsic) pathway is initiated by release of tissue factor (a specific cellular lipoprotein), and can be measured by the prothrombin time (PT) test. PT results are often reported as ratio (INR value) to monitor dosing of oral anticoagulants such as warfarin.
The quantitative and qualitative screening of fibrinogen is measured by the thrombin clotting time (TCT). Measurement of the exact amount of fibrinogen present in the blood is generally done using the Clauss method
for fibrinogen testing. Many analysers are capable of measuring a
"derived fibrinogen" level from the graph of the Prothrombin time clot.
If a coagulation factor is part of the contact activation or
tissue factor pathway, a deficiency of that factor will affect only one
of the tests: Thus hemophilia A,
a deficiency of factor VIII, which is part of the contact activation
pathway, results in an abnormally prolonged aPTT test but a normal PT
test. The exceptions are prothrombin, fibrinogen, and some variants of
FX that can be detected only by either aPTT or PT. If an abnormal PT or
aPTT is present, additional testing will occur to determine which (if
any) factor is present as aberrant concentrations.
Deficiencies of fibrinogen (quantitative or qualitative) will affect all screening tests.
Role in disease
Coagulation defects may cause hemorrhage or thrombosis, and occasionally both, depending on the nature of the defect.
The GP1b-IX receptor complex. This protein receptor complex is found on the surface of platelets, and in conjunction with GPV
allows for platelets to adhere to the site of injury. Mutations in the
genes associated with the glycoprotein Ib-IX-V complex are
characteristic of Bernard–Soulier syndrome
Platelet disorders
Platelet disorders are either congenital or acquired. Examples of congenital platelet disorders are Glanzmann's thrombasthenia, Bernard–Soulier syndrome (abnormal glycoprotein Ib-IX-V complex), gray platelet syndrome (deficient alpha granules), and delta storage pool deficiency (deficient dense granules). Most are rare. They predispose to hemorrhage. Von Willebrand disease is due to deficiency or abnormal function of von Willebrand factor, and leads to a similar bleeding pattern; its milder forms are relatively common.
Decreased platelet numbers (thrombocytopenia) is due to insufficient production (e.g., myelodysplastic syndrome or other bone marrow disorders), destruction by the immune system (immune thrombocytopenic purpura/ITP), or consumption (e.g., thrombotic thrombocytopenic purpura/TTP, hemolytic-uremic syndrome/HUS, paroxysmal nocturnal hemoglobinuria/PNH, disseminated intravascular coagulation/DIC, heparin-induced thrombocytopenia/HIT). Most consumptive conditions lead to platelet activation, and some are associated with thrombosis.
Coagulation factor disorders
The best-known coagulation factor disorders are the hemophilias. The three main forms are hemophilia A (factor VIII deficiency), hemophilia B (factor IX deficiency or "Christmas disease") and hemophilia C
(factor XI deficiency, mild bleeding tendency). Hemophilia A and B are
X-linked recessive disorders, whereas Hemophilia C is a much more rare
autosomal recessive disorder most commonly seen in Ashkenazi Jews.
Von Willebrand disease
(which behaves more like a platelet disorder except in severe cases),
is the most common hereditary bleeding disorder and is characterized as
being inherited autosomal recessive or dominant. In this disease, there
is a defect in von Willebrand factor (vWF), which mediates the binding
of glycoprotein Ib (GPIb) to collagen. This binding helps mediate the
activation of platelets and formation of primary hemostasis.
Bernard–Soulier syndrome is a defect or deficiency in GPIb. GPIb,
the receptor for vWF, can be defective and lead to lack of primary clot
formation (primary hemostasis) and increased bleeding tendency. This is
an autosomal recessive inherited disorder.
Thrombasthenia of Glanzmann and Naegeli (Glanzmann thrombasthenia)
is extremely rare. It is characterized by a defect in GPIIb/IIIa
fibrinogen receptor complex. When GPIIb/IIIa receptor is dysfunctional,
fibrinogen cannot cross-link platelets, which inhibits primary
hemostasis. This is an autosomal recessive inherited disorder.
In liver failure
(acute and chronic forms), there is insufficient production of
coagulation factors by the liver; this may increase bleeding risk.
Deficiency of Vitamin K may also contribute to bleeding disorders because clotting factor maturation depends on Vitamin K.
Thrombosis is the pathological development of blood clots. These clots may break free and become mobile, forming an embolus or grow to such a size that occludes the vessel in which it developed. An embolism is said to occur when the thrombus
(blood clot) becomes a mobile embolus and migrates to another part of
the body, interfering with blood circulation and hence impairing organ
function downstream of the occlusion. This causes ischemia and often leads to ischemic necrosis of tissue. Most cases of venous thrombosis are due to acquired states (older age, surgery, cancer, immobility) or inherited thrombophilias (e.g., antiphospholipid syndrome, factor V Leiden, and various other genetic deficiencies or variants).
Mutations in factor XII have been associated with an asymptomatic prolongation in the clotting time and possibly a tendency toward thrombophlebitis. Other mutations have been linked with a rare form of hereditary angioedema (type III) essentialism.
Pharmacology
Procoagulants
The use of adsorbent chemicals, such as zeolites, and other hemostatic agents
are also used for sealing severe injuries quickly (such as in traumatic
bleeding secondary to gunshot wounds). Thrombin and fibrin glue are used surgically to treat bleeding and to thrombose aneurysms.
Desmopressin is used to improve platelet function by activating arginine vasopressin receptor 1A.
Coagulation factor concentrates are used to treat hemophilia,
to reverse the effects of anticoagulants, and to treat bleeding in
patients with impaired coagulation factor synthesis or increased
consumption. Prothrombin complex concentrate, cryoprecipitate and fresh frozen plasma are commonly used coagulation factor products. Recombinant activated human factor VII is increasingly popular in the treatment of major bleeding.
Tranexamic acid and aminocaproic acid inhibit fibrinolysis and lead to a de facto reduced bleeding rate. Before its withdrawal, aprotinin was used in some forms of major surgery to decrease bleeding risk and need for blood products.
Rivaroxaban drug bound to the coagulation factor Xa. The drug prevents this protein from activating the coagulation pathway by inhibiting its enzymatic activity.
Anticoagulants
Anticoagulants and anti-platelet agents are amongst the most commonly used medications. Anti-platelet agents include aspirin, dipyridamole, ticlopidine, clopidogrel, ticagrelor and prasugrel; the parenteral glycoprotein IIb/IIIa inhibitors are used during angioplasty. Of the anticoagulants, warfarin (and related coumarins) and heparin
are the most commonly used. Warfarin affects the vitamin K-dependent
clotting factors (II, VII, IX, X) and protein C and protein S, whereas
heparin and related compounds increase the action of antithrombin on
thrombin and factor Xa. A newer class of drugs, the direct thrombin inhibitors, is under development; some members are already in clinical use (such as lepirudin).
Also in clinical use are other small molecular compounds that interfere
directly with the enzymatic action of particular coagulation factors
(the directly acting oral anticoagulants: dabigatran, rivaroxaban, apixaban, and edoxaban).






