Uranium mining is the process of extraction of uranium ore from the ground. The worldwide production of uranium in 2019 amounted to 53,656 tonnes. Kazakhstan, Canada, and Australia
were the top three uranium producers, respectively, and together
account for 68% of world production. Other countries producing more than
1,000 tonnes per year included Namibia, Niger, Russia, Uzbekistan, the United States, and China. Nearly all of the world's mined uranium is used to power nuclear power plants. Historically uranium was also used in applications such as uranium glass or ferrouranium
but those applications have declined due to the radioactivity of
uranium and are nowadays mostly supplied with a plentiful cheap supply
of depleted uranium
which is also used in uranium ammunition. In addition to being cheaper,
depleted uranium is also less radioactive due to a lower content of
short-lived 234
U and 235
U than natural uranium.
Uranium is mined by in-situ leaching (57% of world production) or by conventional underground or open-pit mining
of ores (43% of production). During in-situ mining, a leaching solution
is pumped down drill holes into the uranium ore deposit where it
dissolves the ore minerals. The uranium-rich fluid is then pumped back
to the surface and processed to extract the uranium compounds from
solution. In conventional mining, ores are processed by grinding the ore
materials to a uniform particle size and then treating the ore to
extract the uranium by chemical leaching. The milling process commonly yields dry powder-form material consisting of natural uranium, "yellowcake," which is nowadays commonly sold on the uranium market as U3O8. While some nuclear power plants - most notably heavy water reactors like the CANDU - can operate with natural uranium (usually in the form of uranium dioxide), the vast majority of commercial nuclear power plants and many research reactors require uranium enrichment, which raises the content of 235
U from the natural 0.72% to 3-5% (for use in light water reactors) or even higher, depending on the application. Enrichment requires conversion of the yellowcake into uranium hexafluoride and production of the fuel (again usually uranium dioxide, but sometimes uranium carbide, uranium hydride or uranium nitride) from that feedstock.
History
Uranium minerals were noticed by miners for a long time prior to the discovery of uranium in 1789. The uranium mineral pitchblende, also known as uraninite, was reported from the Erzgebirge (Ore Mountains), Saxony, as early as 1565. Other early reports of pitchblende date from 1727 in Jáchymov and 1763 in Schwarzwald. The suffix "-blende" applied by Ore Mountain miners to the mineral long before the discovery of the element uranium indicates a mineral that looks like it contains some usable metal but (according to then available knowledge) doesn't. Terms for minerals later identified to contain cobalt (c.f. "Kobold" the modern standard German word for goblin) nickel or "Wolfram" (the standard German term for tungsten) were similarly derived from superstitious belief that some sort of "mountain gnome" had somehow "hexed" the minerals to trick (c.f. The German use of the term "blenden" cognate of "blind" for "tricking") the miners. Thus examining old documents about the presence of "worthless" -blende type minerals allowed easier exploration, once the true nature of these ores had been discovered.
In the early 19th century, uranium ore was recovered as a byproduct of mining in Saxony, Bohemia, and Cornwall. The first deliberate mining of radioactive ores took place in Joachimsthal, a silver-mining city now in the Czech Republic and origin of the word T(h)aler and from there dollar. Marie Skłodowska-Curie used pitchblende ore from Joachimsthal to isolate the element radium, a decay product
of uranium. This discovery earned her her first Nobel Prize and
represents an important step in the history of nuclear physics. Until
World War II, uranium was mined primarily for its radium content; some carnotite deposits were mined primarily for the vanadium content. Sources for radium, contained in the uranium ore, were sought for use as luminous paint
for watch dials and other instruments, as well as for health-related
applications, some of which in retrospect were certainly harmful and
some of which must be regarded as nuclear quackery even from a contemporary standpoint. Given that the half life of 238
U is almost 2.8 million times longer than that of 226
Ra,
tons of uranium had to be processed to derive a few grams of radium,
leaving large amounts of uranium with little economic uses. The
byproduct uranium was used mostly as a yellow pigment. Ferrouranium started to be used by the Central Powers of World War I
as a substitute for materials they could no longer import due to the
British naval blockade. At the time both sides of the Ore Mountains were
contained in territory controlled by the Central Powers, as the
northern flank belonged to the German Empire whereas the southern flank
belonged to Austria-Hungary.
Given that Uranium was mostly seen as a "waste product" in
radium-mining at the time, a plentiful supply was available even without
scaling up mining. While radium has legitimate evidence based
applications in nuclear medicine, the availability of other radionuclides with more desirable properties has all but eliminated the market for radium.
In the United States, the first radium/uranium ore was discovered in 1871 in gold mines near Central City, Colorado. This district produced about 50 tons of high grade ore between 1871 and 1895. Most American uranium ore before World War II came from vanadium deposits on the Colorado Plateau of Utah and Colorado.
In Cornwall, England, the South Terras Mine near St. Stephen opened for uranium production in 1873, and produced about 175 tons of ore before 1900. Other early uranium mining occurred in Autunois in France's Massif Central (whence the name Autunite for a uranium-bearing mineral once mined in the area), Oberpfalz in Bavaria, and Billingen in Sweden.
The Shinkolobwe deposit in Katanga, Belgian Congo (now Shaba Province, Democratic Republic of the Congo (DRC)), was discovered in 1913, and exploited by the Union Minière du Haut Katanga. Other important deposits mined early in the history of uranium mining include Port Radium, near Great Bear Lake, Canada, discovered in 1931; along with Beira Province, Portugal; Tyuya Muyun, Uzbekistan (whence Tyuyamunite); and Radium Hill, Australia.
Because of the need for the uranium for bomb research during World War II, the Manhattan Project used a variety of sources for the element. The Manhattan Project initially purchased uranium ore from the Belgian Congo, through the Union Minière du Haut Katanga. Later the project contracted with vanadium mining companies in the American Southwest. Purchases were also made from the Eldorado Mining and Refining Limited company in Canada. This company had large stocks of uranium as waste from its radium refining activities.
The smaller scale German nuclear program likewise tried to acquire uranium, putting the Berlin based Auergesellschaft, which had been taken from previous Jewish-German owners, in charge of acquisition. A substantial amount of uranium from the Belgian Congo (and thus the same source as the one used by the Americans) fell into German hands when the Wehrmacht conquered Belgium. Another important source was mining in the Ore Mountains (mostly on the Czech side, which had been annexed after the Munich treaty of 1938). While incompetence, bad luck, lack of resources and infighting kept the Germans from ever assembling enough uranium and neutron moderator in a single "pile" to achieve criticality, by the end of the war more than enough uranium had been in German hands to theoretically allow for the construction of a crude reactor on the scale of Chicago Pile-1 or the Soviet F-1 (nuclear reactor) if it had all been assembled in a single place. The Haigerloch research reactor (de:Forschungsreaktor Haigerloch) assembled months before the end of the war in Southwestern Germany was an extremely subcritical assembly which lacked both sufficient uranium and sufficient moderator to produce significant amounts of plutonium, let alone heat. A uranium cube thought to have belonged to Werner Heisenberg was analyzed decades later, confirming that the Germans were nowhere near plutonium production.
American uranium ores mined in Colorado were mixed ores of vanadium and uranium, but because of wartime secrecy, the Manhattan Project would publicly admit only to purchasing the vanadium, and did not pay the uranium miners for the uranium content. In a much later lawsuit, many miners were able to reclaim lost profits from the U.S. government. American ores had much lower uranium concentrations than the ore from the Belgian Congo, but they were pursued vigorously to ensure nuclear self-sufficiency. Similar efforts were undertaken in the Soviet Union, which did not have native stocks of uranium when it started developing its own atomic weapons program.
Intensive exploration for uranium started after the end of World War II as a result of the military and civilian demand for uranium. There were three separate periods of uranium exploration or "booms." These were from 1956 to 1960, 1967 to 1971, and from 1976 to 1982.
While the Soviet Republics of Kazakhstan and the RSFSR would later become some of the leading uranium producers in the world, immediately after the end of World War II the availability of large uranium deposits in the USSR wasn't yet known and thus the Soviets developed immense mining operations in their satellite states East Germany and Czechoslovakia which had known uranium deposits in the Ore Mountains. The deliberately opaquely named SDAG Wismut (the German term "Wismut" for Bismuth should give the illusion of prospection for a metal the Soviets definitely weren't after) became the biggest employer in the Saxon Ore Mountains and remote mining towns like Johanngeorgenstadt swelled to ten times their population in a few years. The mining cost immense amounts of money and miners were on the one hand subject to heavier repression and surveillance but on the other hand allowed more generous supply with consumer goods than other East Germans. While production was never able to compete with global uranium market prices, the dual use nature of the mined material as well as the possibility to pay miners in soft currency but sell uranium for hard currency or substitute imports which would've had to be paid for in hard currency tipped the scales in favor of continuing mining operations throughout the Cold War. After German reunification, mining was wound down and the arduous task of rehabilitating the land impacted by mining was begun. In the course of this, some remaining deposits had to be mined to reduce the potential harm from material leaching into groundwater, but this has since ceased as well, making the area in which uranium had been discovered two centuries prior entirely devoid of uranium mining.
In the 20th century, the United States was the world's largest uranium producer. Grants Uranium District in northwestern New Mexico was the largest United States uranium producer. The Gas Hills Uranium District was the second largest uranium producer. The famous Lucky Mc Mine is located in the Gas Hills near Riverton, Wyoming. Canada has since surpassed the United States as the cumulative largest producer in the world. In 1990, 55% of world production came from underground mines, but this shrank to 33% by 1999. From 2000, new Canadian mines again increased the proportion of underground mining, and with Olympic Dam it is now 37%. In situ leach (ISL, or ISR) mining has been steadily increasing its share of the total, mainly due to Kazakhstan. Unlike with coal, particularly lignite, where the biggest producers tend to also be the biggest consumers, the biggest uranium producers, Kazakhstan, Australia and Canada contain only one nation – Canada – who derives a significant proportion of electricity from nuclear power. On the other hand, big users of nuclear power like France, South Korea, India or Japan import most or all of the uranium used in their power plants as they have no or negligible domestic uranium resources. India in particular has been interested in the Thorium fuel cycle for decades, as India has much larger thorium reserves than it does uranium reserves. France historically derived significant shares of its uranium needs from its African colonies and continues to be politically and economically active in those African countries to ensure its uranium supply.
Deposit types
Many different types of uranium deposits have been discovered and mined. There are mainly three types of uranium deposits including unconformity-type deposits, namely paleoplacer deposits and sandstone-type also known as roll front type deposits.
Uranium deposits are classified into 15 categories according to their geological setting and the type of rock in which they are found. This geological classification system is determined by the International Atomic Energy Agency (IAEA).
Uranium is also contained in seawater but at present prices on the uranium market, costs would have to be lowered by a factor of 3–6 to make its recovery economical.
Sedimentary
Uranium deposits in sedimentary rocks include those in sandstone (in Canada and the western US), Precambrian unconformities (in Canada), phosphate, Precambrian quartz-pebble conglomerate, collapse breccia pipes (see Arizona breccia pipe uranium mineralization), and calcrete
Sandstone uranium deposits are generally of two types. Roll-front type deposits occur at the boundary between the up dip and oxidized part of a sandstone body and the deeper down dip reduced part of a sandstone body. Peneconcordant sandstone uranium deposits, also called Colorado Plateau-type deposits, most often occur within generally oxidized sandstone bodies, often in localized reduced zones, such as in association with carbonized wood in the sandstone.
Precambrian quartz-pebble conglomerate-type uranium deposits occur only in rocks older than two billion years old. The conglomerates also contain pyrite. These deposits have been mined in the Blind River-Elliot Lake district of Ontario, Canada, and from the gold-bearing Witwatersrand conglomerates of South Africa.
Unconformity-type deposits make up about 33% of the World Outside Centrally Planned Economies Areas (WOCA)'s uranium deposits.
Igneous or hydrothermal
Hydrothermal uranium deposits encompass the vein-type uranium ores. Vein-type hydrothermal uranium deposits represent epigenetic concentrations of uranium minerals that typically fill breccias, fractures, and shear zones. Many studies have sought to identify the source of uranium with hydrothermal vein-type deposits and the potential sources still remains a mystery, but are thought to include preexisting rocks that have been broken down by weathering and force that come from areas of long-term sediment build up. The South Chine Block is an example of a region that has been relying on vein-type hydrothermal uranium deposit demand for the past half century. Igneous deposits include nepheline syenite intrusives at Ilimaussaq, Greenland; the disseminated uranium deposit at Rossing, Namibia; uranium-bearing pegmatites, and the Aurora crater lake deposit of the McDermitt Caldera in Oregon. Disseminated deposits are also found in the states of Washington and Alaska in the US.
Breccia
Breccia uranium deposits are found in rocks that have been broken due to tectonic fracturing, or weathering. Breccia uranium deposits are most common in India, Australia and the United States. A large mass of breccia is called a breccia pipe or chimney and is composed of the rock forming an irregular and almost cylinder like shape. The origin of breccia pipe is uncertain but it is thought that they form on intersections and faults. When the formations are found solid in ground host rock called rock flour, it usually is often a site for copper or uranium mining. Copper Creek, Arizona is home to approximately 500 mineralized breccia pipes and Cripple Creek, Colorado also is a site that contains breccia pipe ore deposits that is associated with a volcanic pipe.
Olympic Dam mine, the world's largest uranium deposit, was discovered by Western Mining Corporation in 1975 and is owned by BHP.
Exploration
Uranium prospecting is similar to other forms of mineral exploration with the exception of some specialized instruments for detecting the presence of radioactive isotopes.
The Geiger counter was the original radiation detector, recording the total count rate from all energy levels of radiation. Ionization chambers and Geiger counters were first adapted for field use in the 1930s. The first transportable Geiger–Müller counter (weighing 25 kg) was constructed at the University of British Columbia in 1932. H.V. Ellsworth of the GSC built a lighter weight, more practical unit in 1934. Subsequent models were the principal instruments used for uranium prospecting for many years, until geiger counters were replaced by scintillation counters.
The use of airborne detectors to prospect for radioactive minerals was first proposed by G.C. Ridland, a geophysicist working at Port Radium in 1943. In 1947, the earliest recorded trial of airborne radiation detectors (ionization chambers and Geiger counters) was conducted by Eldorado Mining and Refining Limited. (a Canadian Crown Corporation since sold to become Cameco Corporation). The first patent for a portable gamma-ray spectrometer was filed by Professors Pringle, Roulston & Brownell of the University of Manitoba in 1949, the same year as they tested the first portable scintillation counter on the ground and in the air in northern Saskatchewan.
Airborne gamma-ray spectrometry is now the accepted leading technique for uranium prospecting with worldwide applications for geological mapping, mineral exploration & environmental monitoring. Airborne gamma-ray spectrometry used specifically for uranium measurement and prospecting must account for a number of factors like the distance between the source and the detector and the scattering of radiation through the minerals, surrounding earth and even in the air. In Australia, a Weathering Intensity Index has been developed to help prospectors based on the Shuttle Radar Topography Mission (SRTM) elevation and airborne gamma-ray spectrometry images.
A deposit of uranium, discovered by geophysical techniques, is evaluated and sampled to determine the amounts of uranium materials that are extractable at specified costs from the deposit. Uranium reserves are the amounts of ore that are estimated to be recoverable at stated costs. As prices rise or technology allows for lower cost of recovery of known, previously uneconomic, deposits, reserves increase. For uranium this effect is particularly pronounced as the biggest currently uneconomic reserve – uranium extraction from seawater – is bigger than all known land based resources of uranium combined.
The OECD's red book of 2011 reported that conventional uranium resources had grown by 12.5% since 2008 due to increased exploration.
Mining techniques
As with other types of hard rock mining there are several methods of extraction. In 2016, the percentage of the mined uranium produced by each mining method was: in-situ leach (49.7 percent), underground mining (30.8 percent), open pit (12.9 percent), heap leaching (0.4 percent), co-product/by-product (6.1%). The remaining 0,1% was derived as miscellaneous recovery.
Open pit
In open pit mining, overburden is removed by drilling and blasting to expose the ore body, which is then mined by blasting and excavation using loaders and dump trucks. Workers spend much time in enclosed cabins thus limiting exposure to radiation. Water is extensively used to suppress airborne dust levels. Groundwater is an issue in all types of mining, but in open pit mining, the usual way of dealing with it - i.e. when the target mineral is found below the natural water table - is to lower the water table by pumping off the water. The ground may settle considerably when groundwater is removed and may again move unpredictably when groundwater is allowed to rise again after mining is concluded. Land reclamation after mining takes different routes, depending on the amount of material removed. Due to the high energy density of uranium, it is often sufficient to fill in the former mine with the overburden, but in case of a mass deficit exceeding the height difference between the previous surface level and the natural water table, artificial lakes develop when groundwater removal ceases. If sulfites, sulfides or sulfates are present in the now-exposed rocks acid mine drainage can be a concern for those newly developing bodies of water. Mining companies are now required by law to establish a fund for future reclamation while mining is ongoing and those funds are usually deposited in such a way as to be unaffected by bankruptcy of the mining company.
Underground
If the uranium is too far below the surface for open pit mining, an underground mine might be used with tunnels and shafts dug to access and remove uranium ore.
Underground uranium mining is in principle no different from any other hard rock mining and other ores are often mined in association (e.g., copper, gold, silver). Once the ore body has been identified a shaft is sunk in the vicinity of the ore veins, and crosscuts are driven horizontally to the veins at various levels, usually every 100 to 150 metres. Similar tunnels, known as drifts, are driven along the ore veins from the crosscut. To extract the ore, the next step is to drive tunnels, known as raises when driven upwards and winzes when driven downwards, through the deposit from level to level. Raises are subsequently used to develop the stopes where the ore is mined from the veins.
The stope, which is the workshop of the mine, is the excavation from which the ore is extracted. Three methods of stope mining are commonly used. In the "cut and fill" or "open stoping" method, the space remaining following removal of ore after blasting is filled with waste rock and cement. In the "shrinkage" method, only sufficient broken ore is removed via the chutes below to allow miners working from the top of the pile to drill and blast the next layer to be broken off, eventually leaving a large hole. The method known as "room and pillar" is used for thinner, flatter ore bodies. In this method the ore body is first divided into blocks by intersecting drives, removing ore while so doing, and then systematically removing the blocks, leaving enough ore for roof support.
The health effects discovered from radon exposure in unventilated uranium mining prompted the switch away from uranium mining via tunnel mining towards open cut and in-situ leaching technology, a method of extraction that does not produce the same occupational hazards, or mine tailings, as conventional mining.
With regulations in place to ensure the use of high volume ventilation technology if any confined space uranium mining is occurring, occupational exposure and mining deaths can be largely eliminated. The Olympic Dam and Canadian underground mines are ventilated with powerful fans with radon levels being kept at a very low to practically "safe level" in uranium mines. Naturally occurring radon in other, non-uranium mines, also may need control by ventilation.
Heap leaching
Heap leaching is an extraction process by which chemicals (usually sulfuric acid) are used to extract the economic element from ore which has been mined and placed in piles on the surface. Heap leaching is generally economically feasible only for oxide ore deposits. Oxidation of sulfide deposits occurs during the geological process called weathering. Therefore, oxide ore deposits are typically found close to the surface. If there are no other economic elements within the ore a mine might choose to extract the uranium using a leaching agent, usually a low molar sulfuric acid.
If the economic and geological conditions are right, the mining company will level large areas of land with a small gradient, layering it with thick plastic (usually HDPE or LLDPE), sometimes with clay, silt or sand beneath the plastic liner. The extracted ore will typically be run through a crusher and placed in heaps atop the plastic. The leaching agent will then be sprayed on the ore for 30–90 days. As the leaching agent filters through the heap, the uranium will break its bonds with the oxide rock and enter the solution. The solution will then filter along the gradient into collecting pools which will then be pumped to on-site plants for further processing. Only some of the uranium (commonly about 70%) is actually extracted.
The uranium concentrations within the solution are very important for the efficient separation of pure uranium from the acid. As different heaps will yield different concentrations, the solution is pumped to a mixing plant that is carefully monitored. The properly balanced solution is then pumped into a processing plant where the uranium is separated from the sulfuric acid.
Heap leach is significantly cheaper than traditional milling processes. The low costs allow for lower grade ore to be economically feasible (given that it is the right type of ore body). Environmental law requires that the surrounding ground water is continually monitored for possible contamination. The mine will also have to have continued monitoring even after the shutdown of the mine. In the past mining companies would sometimes go bankrupt, leaving the responsibility of mine reclamation to the public. Recent additions to the mining law require that companies set aside the money for reclamation before the beginning of the project. The money will be held by the public to insure adherence to environmental standards if the company were to ever go bankrupt.
In-situ leaching
In-situ leaching (ISL), also known as solution mining, or in-situ recovery (ISR) in North America, involves leaving the ore where it is in the ground, and recovering the minerals from it by dissolving them and pumping the pregnant solution to the surface where the minerals can be recovered. Consequently, there is little surface disturbance and no tailings or waste rock generated. However, the orebody needs to be permeable to the liquids used, and located so that they do not contaminate ground water away from the orebody.
Uranium ISL uses the native groundwater in the orebody which is fortified with a complexing agent and in most cases an oxidant. It is then pumped through the underground orebody to recover the minerals in it by leaching. Once the pregnant solution is returned to the surface, the uranium is recovered in much the same way as in any other uranium plant (mill).
In Australian ISL mines (Beverley, Four Mile and Honeymoon Mine) the oxidant used is hydrogen peroxide and the complexing agent sulfuric acid. Kazakh ISL mines generally do not employ an oxidant but use much higher acid concentrations in the circulating solutions. ISL mines in the USA use an alkali leach due to the presence of significant quantities of acid-consuming minerals such as gypsum and limestone in the host aquifers. Any more than a few percent carbonate minerals means that alkali leach must be used in preference to the more efficient acid leach.
The Australian government has published a best practice guide for in situ leach mining of uranium, which is being revised to take account of international differences.
Seawater recovery
The uranium concentration in sea water is low, approximately 3.3 parts per billion or 3.3 micrograms per liter of seawater. But the quantity of this resource is gigantic and some scientists believe this resource is practically limitless with respect to world-wide demand. That is to say, if even a portion of the uranium in seawater could be used the entire world's nuclear power generation fuel could be provided over a long time period. Some anti-nuclear proponents claim this statistic is exaggerated. Although research and development for recovery of this low-concentration element by inorganic adsorbents such as titanium oxide compounds has occurred since the 1960s in the United Kingdom, France, Germany, and Japan, this research was halted due to low recovery efficiency.
At the Takasaki Radiation Chemistry Research Establishment of the Japan Atomic Energy Research Institute (JAERI Takasaki Research Establishment), research and development has continued culminating in the production of adsorbent by irradiation of polymer fiber. Adsorbents have been synthesized that have a functional group (amidoxime group) that selectively adsorbs heavy metals, and the performance of such adsorbents has been improved. Uranium adsorption capacity of the polymer fiber adsorbent is high, approximately tenfold greater in comparison to the conventional titanium oxide adsorbent.
One method of extracting uranium from seawater is using a uranium-specific nonwoven fabric as an adsorbent. The total amount of uranium recovered from three collection boxes containing 350 kg of fabric was >1 kg of yellowcake after 240 days of submersion in the ocean. According to the OECD, uranium may be extracted from seawater using this method for about $300/kg-U. The experiment by Seko et al. was repeated by Tamada et al. in 2006. They found that the cost varied from ¥15,000 to ¥88,000 depending on assumptions and "The lowest cost attainable now is ¥25,000 with 4g-U/kg-adsorbent used in the sea area of Okinawa, with 18 repetitionuses [sic]." With the May, 2008 exchange rate, this was about $240/kg-U.
In 2012, ORNL researchers announced the successful development of a new adsorbent material dubbed "HiCap", which vastly outperforms previous best adsorbents, which perform surface retention of solid or gas molecules, atoms or ions. "We have shown that our adsorbents can extract five to seven times more uranium at uptake rates seven times faster than the world's best adsorbents," said Chris Janke, one of the inventors and a member of ORNL's Materials Science and Technology Division. HiCap also effectively removes toxic metals from water, according to results verified by researchers at Pacific Northwest National Laboratory.
In 2012 it was estimated that this fuel source could be extracted at 10 times the current price of uranium. In 2014, with the advances made in the efficiency of seawater uranium extraction, it was suggested that it would be economically competitive to produce fuel for light water reactors from seawater if the process was implemented at large scale. Uranium extracted on an industrial scale from seawater would constantly be replenished by both river erosion of rocks and the natural process of uranium dissolved from the surface area of the ocean floor, both of which maintain the solubility equilibria of seawater concentration at a stable level. Some commentators have argued that this strengthens the case for nuclear power to be considered a renewable energy.
Co-product/by-product
Uranium can be recovered as a by-product along with other co-products such as molybdenum, vanadium, nickel, zinc and petroleum products. Uranium is also often found in phosphate minerals, where it has to be removed because phosphate is mostly used for fertilizers. Phosphogypsum is a waste product from phosphate mining that can contain significant amounts of uranium and radium. Coal fly ash also contains significant amounts of uranium and has been suggested as a source for uranium extraction.
Uranium prices
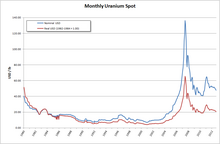
Generally speaking, in the case of nuclear energy the cost of fuel has the lowest share in total energy costs of all fuel consuming energy forms (i.e. Fossil fuels, biomass and nuclear). Furthermore, given the immense energy density of nuclear fuel (particularly in the form of enriched uranium or high grade plutonium), it is easy to stockpile amounts of fuel material to last several years at constant consumption. Power plants that do not have online refuelling capabilities, as is the case for the vast majority of commercial power plants in operation, will refuel as seldom as possible to avoid costly downtime and usually plan refuelling shutdowns long in advance so as to allow maintenance and inspection to use the scheduled downtime as well. As such power plant operators tend to have long-term contracts with fuel suppliers that are – if at all – only minorly affected by the fluctuations of uranium prices. The effect on electricity price for end consumers is negligible even in countries like France, which derive a majority of their electric energy from nuclear power. Nonetheless, short term price developments like the 2007 uranium bubble, can have drastic effects on mining companies, prospection and the economic calculations as to whether a certain deposit is worthwhile for commercial purposes.
Since 1981 uranium prices and quantities in the US are reported by the Department of Energy. The import price dropped from 32.90 US$/lb-U3O8 in 1981 down to 12.55 in 1990 and to below 10 US$/lb-U3O8 in the year 2000. Prices paid for uranium during the 1970s were higher, 43 US$/lb-U3O8 is reported as the selling price for Australian uranium in 1978 by the Nuclear Information Centre. Uranium prices reached an all-time low in 2001, costing US$7/lb, but in April 2007 the price of Uranium on the spot market rose to US$113.00/lb, a high point of the uranium bubble of 2007. This was very close to the all time high (adjusted for inflation) in 1977.
Following the 2011 Fukushima nuclear disaster, the global uranium sector remained depressed with the uranium price falling more than 50%, declining share values, and reduced profitability of uranium producers since March 2011 and into 2014. As a result, uranium companies worldwide are reducing costs, and limiting operations. As an example, Westwater Resources (previously Uranium Resources), has had to cease all uranium operations due to unfavorable prices. Since then, Westwater has tried branching out into other markets, namely lithium and graphite.
As of July 2014, the price of uranium concentrate remained near a five-year low, the uranium price having fallen more than 50% from the peak spot price in January 2011, reflecting the loss of Japanese demand following the 2011 Fukushima nuclear disaster. As a result of continued low prices, in February 2014 mining company Cameco deferred plans to expand production from existing Canadian mines, although it continued work to open a new mine at Cigar Lake. Also in February 2014, Paladin energy suspended operations at its mine in Malawi, saying that the high-cost operation was losing money at current prices.
Effect of the uranium price on mining and nuclear power plants
In general short term fluctuations in the price of uranium are of more concern to operators and owners of mines and potentially lucrative deposits than to power plant operators. Due to its high energy density, uranium is easy to stockpile in the form of strategic reserves and thus a short term increase in prices can be compensated by accessing those reserves. Furthermore, many countries have de facto reserves in the form of reprocessed uranium or depleted uranium which still contain a share of fissile material that can make re-enrichment worthwhile if market conditions call for it. Nuclear reprocessing of spent fuel is - as of the 2020s - done commercially primarily to use the fissile material still contained in spent fuel. The commonly employed PUREX process recovers uranium and plutonium which can then be converted into MOX-fuel for use in the same light water reactors that produced the spent fuel. Whether reprocessing is economical is subject to much debate and depends in part on assumptions as to the price of uranium and the cost of disposal via deep geological repository or nuclear transmutation. Reactors that can run on natural uranium consume less mined uranium per unit of power produced but can have higher capital costs to build due to the need for heavy water as moderator. Furthermore they need to be capable of online refueling because the burnup achievable with natural uranium is lower than that achievable with enriched uranium - having to shut down the entire reactor for every refueling would quickly make such a reactor uneconomic. Breeder reactors also become more economical as uranium prices rise and it was among other things a decline in uranium prices in the 1970s that led to a decline in interest in breeder reactor technology. The thorium fuel cycle is a further alternative if and when uranium prices remain at a sustained high level and consequently interest in this alternative to current "mainstream" light water reactor technology is dependent in no small part on uranium prices.
Politics
In the beginning of the Cold War, to ensure adequate supplies of uranium for national defense, the United States Congress passed the U.S. Atomic Energy Act of 1946, creating the Atomic Energy Commission (AEC) which had the power to withdraw prospective uranium mining land from public purchase, and also to manipulate the price of uranium to meet national needs. By setting a high price for uranium ore, the AEC created a uranium "boom" in the early 1950s, which attracted many prospectors to the Four Corners region of the country. Moab, Utah became known as the Uranium-capital of the world, when geologist Charles Steen discovered such an ore in 1952, even though American ore sources were considerably less potent than those in the Belgian Congo or South Africa.
In the 1950s methods for extracting diluted uranium and thorium, found in abundance in granite or seawater, were pursued. Scientists speculated that, used in a breeder reactor, these materials would potentially provide limitless source of energy.
American military requirements declined in the 1960s, and the government completed its uranium procurement program by the end of 1970. Simultaneously, a new market emerged: commercial nuclear power plants. In the U.S. this market virtually collapsed by the end of the 1970s as a result of industrial strains caused by the energy crisis, popular opposition, and finally the Three Mile Island nuclear accident in 1979, all of which led to a de facto moratorium on the development of new nuclear reactor power stations.
In Europe a mixed situation exists. Considerable nuclear power capacities have been developed, notably in Belgium, Finland, France, Germany, Spain, Sweden, Switzerland, and the UK. In many countries development of nuclear power has been stopped and phased out by legal actions. In Italy the use of nuclear power was barred by a referendum in 1987; this is now under revision. Ireland in 2008 also had no plans to change its non-nuclear stance.
The years 1976 and 1977 saw uranium mining become a major political issue in Australia, with the Ranger Inquiry (Fox) report opening up a public debate about uranium mining. The Movement Against Uranium Mining group was formed in 1976, and many protests and demonstrations against uranium mining were held. Concerns relate to the health risks and environmental damage from uranium mining. Notable Australian anti-uranium activists have included Kevin Buzzacott, Jacqui Katona, Yvonne Margarula, and Jillian Marsh.
The World Uranium Hearing was held in Salzburg, Austria in September 1992. Anti-nuclear speakers from all continents, including indigenous speakers and scientists, testified to the health and environmental problems of uranium mining and processing, nuclear power, nuclear weapons, nuclear tests, and radioactive waste disposal. People who spoke at the 1992 Hearing include: Thomas Banyacya, Katsumi Furitsu, Manuel Pino and Floyd Red Crow Westerman. They highlighted the threat of radioactive contamination to all peoples, especially indigenous communities and said that their survival requires self-determination and emphasis on spiritual and cultural values. Increased renewable energy commercialization was advocated.
The Kingdom of Saudi Arabia with the help of China has built an extraction facility to obtain uranium yellowcake from uranium ore. According to Western officials with information regarding the extraction site, the process is conducted by the oil-rich kingdom to champion nuclear technology. However, Saudi Energy Minister denied having built a uranium ore facility and claimed that the extraction of minerals is a fundamental part of the kingdom’s strategy to diversify its economy.
Health risks
Uranium ore emits radon gas. The health effects of high exposure to radon are a particular problem in the mining of uranium; significant excess lung cancer deaths have been identified in epidemiological studies of uranium miners employed in the 1940s and 1950s.
The first major studies with radon and health occurred in the context of uranium mining, first in the Joachimsthal region of Bohemia and then in the Southwestern United States during the early Cold War. Because radon is a product of the radioactive decay of uranium, underground uranium mines may have high concentrations of radon. Many uranium miners in the Four Corners region contracted lung cancer and other pathologies as a result of high levels of exposure to radon in the mid-1950s. The increased incidence of lung cancer was particularly pronounced among Native American and Mormon miners, because those groups normally have low rates of lung cancer. This is in part due to the religious prohibition on smoking in Mormonism. Safety standards requiring expensive ventilation were not widely implemented or policed during this period. While radon exposure is the main source of lung cancer in non-smokers who aren't exposed to asbestos, there is evidence that the combination of smoking and radon exposure increases the risk above the combined risks of either harmful substance.
In studies of uranium miners, workers exposed to radon levels of 50 to 150 picocuries of radon per liter of air (2000–6000 Bq/m3) for about 10 years have shown an increased frequency of lung cancer. Statistically significant excesses in lung cancer deaths were present after cumulative exposures of less than 50 WLM. There is unexplained heterogeneity in these results (whose confidence interval do not always overlap). The size of the radon-related increase in lung cancer risk varied by more than an order of magnitude between the different studies.
Since that time, ventilation and other measures have been used to reduce radon levels in most affected mines that continue to operate. In recent years, the average annual exposure of uranium miners has fallen to levels similar to the concentrations inhaled in some homes. This has reduced the risk of occupationally induced cancer from radon, although it still remains an issue both for those who are currently employed in affected mines and for those who have been employed in the past. The power to detect any excess risks in miners nowadays is likely to be small, exposures being much smaller than in the early years of mining. Coal mining in addition to other health risks can also expose miners to radon as Uranium (and its decay product radon) are often found in and near coal deposits and can accumulate underground as radon is denser than air.
In the USA, the Radiation Exposure Compensation Act provides compensation to sufferers of various health problems linked to radiation exposure, or to their surviving relatives. Uranium miners, uranium mill workers and uranium transport workers have been compensated under the scheme.
United States clean-up efforts
Despite efforts made in cleaning up uranium sites, significant problems stemming from the legacy of uranium development still exist today on the territory of the Navajo Nation and in the states of Utah, Colorado, New Mexico, and Arizona. Hundreds of abandoned mines have not been cleaned up and present environmental and health risks in many communities. At the request of the U.S. House Committee on Oversight and Government Reform in October 2007, and in consultation with the Navajo Nation, the Environmental Protection Agency (EPA), along with the Bureau of Indian Affairs (BIA), the Nuclear Regulatory Commission (NRC), the Department of Energy (DOE), and the Indian Health Service (IHS), developed a coordinated Five-Year Plan to address uranium contamination. Similar interagency coordination efforts are beginning in the State of New Mexico as well. In 1978, Congress passed the Uranium Mill Tailings Radiation Control Act (UMTRCA), a measure designed to assist in the cleanup of 22 inactive ore-processing sites throughout the southwest. This also included constructing 19 disposal sites for the tailings, which contain a total of 40 million cubic yards of low-level radioactive material. The Environmental Protection Agency estimates that there are 4000 mines with documented uranium production, and another 15,000 locations with uranium occurrences in 14 western states, most found in the Four Corners area and Wyoming.
The Uranium Mill Tailings Radiation Control Act is a United States environmental law that amended the Atomic Energy Act of 1954 and gave the Environmental Protection Agency the authority to establish health and environmental standards for the stabilization, restoration, and disposal of uranium mill tailings. Title 1 of the Act required the EPA to set environmental protection standards consistent with the Resource Conservation and Recovery Act, including groundwater protection limits; the Department of Energy to implement EPA standards and provide perpetual care for some sites; and the Nuclear Regulatory Commission to review cleanups and license sites to states or the DOE for perpetual care. Title 1 established a uranium mill remedial action program jointly funded by the federal government and the state. Title 1 of the Act also designated 22 inactive uranium mill sites for remediation, resulting in the containment of 40 million cubic yards of low-level radioactive material in UMTRCA Title 1 holding cells.