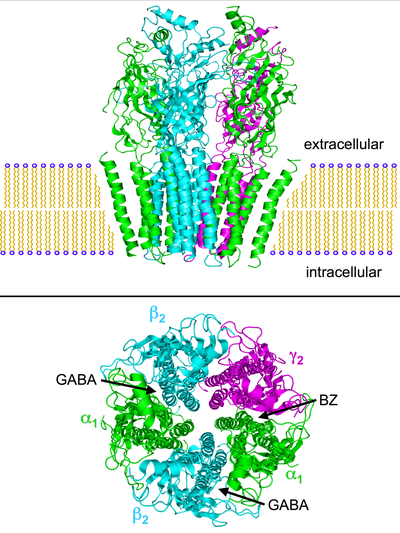
Structure of the nicotinic acetylcholine receptor (nAchR: PDB: 2BG9) which is very similar to the GABAA receptor. Top: side view of the nAchR embedded in a cell membrane. Bottom: view of the receptor from the extracellular face of the membrane. The subunits are labeled according to the GABAA
nomenclature and the approximate locations of the GABA and
benzodiazepine (BZ) binding sites are noted (between the α- and
β-subunits and between the α- and γ-subunits respectively).
Schematic structure of the GABAA receptor. Left: GABAA monomeric subunit imbedded in a lipid bilayer (yellow lines connected to blue spheres). The four transmembrane α-helices
(1–4) are depicted as cylinders. The disulfide bond in the N-terminal
extracellular domain which is characteristic of the family of cys-loop receptors (which includes the GABAA receptor) is depicted as a yellow line. Right:
Five subunits symmetrically arranged about the central chloride anion
conduction pore. The extracellular loops are not depicted for the sake
of clarity.
The GABAA receptor (GABAAR) is an ionotropic receptor and ligand-gated ion channel. Its endogenous ligand is γ-aminobutyric acid (GABA), the major inhibitory neurotransmitter in the central nervous system. Upon activation, the GABAA receptor selectively conducts Cl− through its pore. Cl- will flow out of the cell if the internal voltage is less than resting potential and Cl- will flow in if it is more than resting potential (i.e. -75mV). This causes an inhibitory effect on neurotransmission by diminishing the chance of a successful action potential occurring. The reversal potential of the GABAA-mediated inhibitory postsynaptic potential (IPSP) in normal solution is −70 mV, contrasting the GABAB IPSP (-100mV).
The active site of the GABAA receptor is the binding site for GABA and several drugs such as muscimol, gaboxadol, and bicuculline. The protein also contains a number of different allosteric binding sites which modulate the activity of the receptor indirectly. These allosteric sites are the targets of various other drugs, including the benzodiazepines, nonbenzodiazepines, neuroactive steroids, barbiturates, alcohol (ethanol), inhaled anaesthetics, and picrotoxin, among others.
GABAA receptors occur in all organisms that have a nervous system. To a limited extent the receptors can be found in non-neuronal tissues. Due to their wide distribution within the nervous system of mammals they play a role in virtually all brain functions.
Target for benzodiazepines
The ionotropic GABAA receptor protein complex is also the molecular target of the benzodiazepine class of tranquilizer drugs. Benzodiazepines do not bind to the same receptor site on the protein complex as the endogenous ligand GABA (whose binding site is located between α- and β-subunits), but bind to distinct benzodiazepine binding sites situated at the interface between the α- and γ-subunits of α- and γ-subunit containing GABAA receptors. While the majority of GABAA receptors (those containing α1-, α2-, α3-, or α5-subunits) are benzodiazepine sensitive, there exists a minority of GABAA receptors (α4- or α6-subunit containing) which are insensitive to classical 1,4-benzodiazepines, but instead are sensitive to other classes of GABAergic drugs such as neurosteroids and alcohol. In addition peripheral benzodiazepine receptors exist which are not associated with GABAA receptors. As a result, the IUPHAR has recommended that the terms "BZ receptor", "GABA/BZ receptor" and "omega receptor" no longer be used and that the term "benzodiazepine receptor" be replaced with "benzodiazepine site".In order for GABAA receptors to be sensitive to the action of benzodiazepines they need to contain an α and a γ subunit, between which the benzodiazepine binds. Once bound, the benzodiazepine locks the GABAA receptor into a conformation where the neurotransmitter GABA has much higher affinity for the GABAA receptor, increasing the frequency of opening of the associated chloride ion channel and hyperpolarising the membrane. This potentiates the inhibitory effect of the available GABA leading to sedative and anxiolytic effects.
Different benzodiazepines have different affinities for GABAA receptors made up of different collection of subunits, and this means that their pharmacological profile varies with subtype selectivity. For instance, benzodiazepine receptor ligands with high activity at the α1 and/or α5 tend to be more associated with sedation, ataxia and amnesia, whereas those with higher activity at GABAA receptors containing α2 and/or α3 subunits generally have greater anxiolytic activity. Anticonvulsant effects can be produced by agonists acting at any of the GABAA subtypes, but current research in this area is focused mainly on producing α2-selective agonists as anticonvulsants which lack the side effects of older drugs such as sedation and amnesia.
The binding site for benzodiazepines is distinct from the binding site for barbiturates and GABA on the GABAA receptor, and also produces different effects on binding, with the benzodiazepines causing bursts of chloride channel opening to occur more often, while the barbiturates cause the duration of bursts of chloride channel opening to become longer. Since these are separate modulatory effects, they can both take place at the same time, and so the combination of benzodiazepines with barbiturates is strongly synergistic, and can be dangerous if dosage is not strictly controlled.
Also note that some GABAA agonists such as muscimol and gaboxadol do bind to the same site on the GABAA receptor complex as GABA itself, and consequently produce effects which are similar but not identical to those of positive allosteric modulators like benzodiazepines.
Structure and function
Schematic diagram of a GABAA receptor protein ((α1)2(β2)2(γ2)) which illustrates the five combined subunits that form the protein, the chloride (Cl−)
ion channel pore, the two GABA active binding sites at the α1 and β2
interfaces, and the benzodiazepine (BDZ) allosteric binding site
GABAA receptors are pentameric transmembrane receptors which consist of five subunits arranged around a central pore. Each subunit comprises four transmembrane domains with both the N- and C-terminus located extracellularly. The receptor sits in the membrane of its neuron, usually localized at a synapse, postsynaptically. However, some isoforms may be found extrasynaptically. The ligand GABA is the endogenous compound that causes this receptor to open; once bound to GABA, the protein receptor changes conformation within the membrane, opening the pore in order to allow chloride anions (Cl−) to pass down an electrochemical gradient. Because the reversal potential for chloride in most neurons is close to or more negative than the resting membrane potential, activation of GABAA receptors tends to stabilize or hyperpolarise the resting potential, and can make it more difficult for excitatory neurotransmitters to depolarize the neuron and generate an action potential. The net effect is typically inhibitory, reducing the activity of the neuron. However, depolarizing responses have been found to occur in response to GABA in immature neurons due to a modified Cl- gradient. These depolarization events have shown to be key in neuronal development. In the mature neuron, the GABAA channel opens quickly and thus contributes to the early part of the inhibitory post-synaptic potential (IPSP). The endogenous ligand that binds to the benzodiazepine site is inosine.
Subunits
GABAA receptors are members of the large pentameric ligand gated ion channel (previously referred to as "Cys-loop" receptors) super-family of evolutionarily related and structurally similar ligand-gated ion channels that also includes nicotinic acetylcholine receptors, glycine receptors, and the 5HT3 receptor. There are numerous subunit isoforms for the GABAA receptor, which determine the receptor's agonist affinity, chance of opening, conductance, and other properties.In humans, the units are as follows:
- six types of α subunits (GABRA1, GABRA2, GABRA3, GABRA4, GABRA5, GABRA6)
- three βs (GABRB1, GABRB2, GABRB3)
- three γs (GABRG1, GABRG2, GABRG3)
- as well as a δ (GABRD), an ε (GABRE), a π (GABRP), and a θ (GABRQ)
Distribution
GABAA receptors are responsible for most of the physiological activities of GABA in the central nervous system, and the receptor subtypes vary significantly. Subunit composition can vary widely between regions and subtypes may be associated with specific functions. The minimal requirement to produce a GABA-gated ion channel is the inclusion of an α and a β subunit. The most common GABAA receptor is a pentamer comprising two α's, two β's, and a γ (α1β2γ2). In neurons themselves, the type of GABAA receptor subunits and their densities can vary between cell bodies and dendrites. GABAA receptors can also be found in other tissues, including leydig cells, placenta, immune cells, liver, bone growth plates and several other endocrine tissues. Subunit expression varies between 'normal' tissue and malignancies, as GABAA receptors can influence cell proliferation.Distribution of Receptor Types
| Isoform | Synaptic/Extrasynaptic | Anatomical location |
|---|---|---|
| α1β3γ2S | Both | Widespread |
| α2β3γ2S | Both | Widespread |
| α3β3γ2S | Both | Reticular thalamic nucleus |
| α4β3γ2S | Both | Thalamic relay cells |
| α5β3γ2S | Both | Hippocampal pyramidal cells |
| α6β3γ2S | Both | Cerebellar granule cells |
| α1β2γ2S | Both | Widespread, most abundant |
| α4β3δ | Extrasynaptic | Thalamic relay cells |
| α6β3δ | Extrasynaptic | Cerebellar granule cells |
| α1β2 | Extrasynaptic | Widespread |
| α1β3 | Extrasynaptic | Thalamus, hypothalamus |
| α1β2δ | Extrasynaptic | Hippocampus |
| α4β2δ | Extrasynaptic | Hippocampus |
| α3β3θ | Extrasynaptic | Hypothalamus |
| α3β3ε | Extrasynaptic | Hypothalamus |
Ligands
GABAA receptor and where various ligands bind.
A number of ligands have been found to bind to various sites on the GABAA receptor complex and modulate it besides GABA itself. A ligand can possess one or more properties of the following types. Unfortunately the literature often does not distinguish these types properly.
Types
- Orthosteric agonists and antagonists: bind to the main receptor site (the site where GABA normally binds, also referred to as the "active" or "orthosteric" site). Agonists activate the receptor, resulting in increased Cl− conductance. Antagonists, though they have no effect on their own, compete with GABA for binding and thereby inhibit its action, resulting in decreased Cl− conductance.
- Allosteric agonists: bind to allosteric sites on the receptor and activate the receptor in absence of orthosteric ligands.
- First order allosteric modulators: bind to allosteric sites on the receptor complex and affect it either in a positive (PAM), negative (NAM) or neutral/silent (SAM) manner, causing increased or decreased efficiency of the main site and therefore an indirect increase or decrease in Cl− conductance. SAMs do not affect the conductance, but occupy the binding site.
- Second order modulators: bind to an allosteric site on the receptor complex and modulate the effect of first order modulators.
- Open channel blockers: prolong ligand-receptor occupancy, activation kinetics and Cl ion flux in a subunit configuration-dependent and sensitization-state dependent manner.
- Non-competitive channel blockers: bind to or near the central pore of the receptor complex and directly block Cl− conductance through the ion channel.
Examples
- Orthosteric agonists: GABA, gaboxadol, isoguvacine, muscimol, progabide, piperidine-4-sulfonic acid (partial agonist).
- Orthosteric antagonists: bicuculline, gabazine.
- Positive allosteric modulators: barbiturates, benzodiazepines, certain carbamates (ex. carisoprodol, meprobamate, lorbamate), thienodiazepines, alcohol (ethanol), etomidate, glutethimide, kavalactones, meprobamate, quinazolinones (ex. methaqualone, etaqualone, diproqualone), neuroactive steroids, niacin/niacinamide, nonbenzodiazepines (ex. zolpidem, eszopiclone), propofol, stiripentol, theanine, valerenic acid, volatile/inhaled anesthetics, lanthanum, and riluzole.
- Negative allosteric modulators: flumazenil, Ro15-4513, sarmazenil, amentoflavone, and zinc.
- Second-order modulators: (−)‐epigallocatechin‐3‐gallate.
- Non-competitive channel blockers: cicutoxin, oenanthotoxin, pentylenetetrazol, picrotoxin, thujone, and lindane.
Effects
Ligands which contribute to receptor activation typically have anxiolytic, anticonvulsant, amnesic, sedative, hypnotic, euphoriant, and muscle relaxant properties. Some such as muscimol and the z-drugs may also be hallucinogenic. Ligands which decrease receptor activation usually have opposite effects, including anxiogenesis and convulsion.[citation needed] Some of the subtype-selective negative allosteric modulators such as α5IA are being investigated for their nootropic effects, as well as treatments for the unwanted side effects of other GABAergic drugs.Novel drugs
A useful property of the many benzodiazepine site allosteric modulators is that they may display selective binding to particular subsets of receptors comprising specific subunits. This allows one to determine which GABAA receptor subunit combinations are prevalent in particular brain areas and provides a clue as to which subunit combinations may be responsible for behavioral effects of drugs acting at GABAA receptors. These selective ligands may have pharmacological advantages in that they may allow dissociation of desired therapeutic effects from undesirable side effects. Few subtype selective ligands have gone into clinical use as yet, with the exception of zolpidem which is reasonably selective for α1, but several more selective compounds are in development such as the α3-selective drug adipiplon. There are many examples of subtype-selective compounds which are widely used in scientific research, including:- CL-218,872 (highly α1-selective agonist)
- bretazenil (subtype-selective partial agonist)
- imidazenil and L-838,417 (both partial agonists at some subtypes, but weak antagonists at others)
- QH-ii-066 (full agonist highly selective for α5 subtype)
- α5IA (selective inverse agonist for α5 subtype)
- SL-651,498 (full agonist at α2 and α3 subtypes, and as a partial agonist at α1 and α5
- 3-acyl-4-quinolones: selective for α1 over α3