In thermodynamics, the Gibbs free energy (IUPAC recommended name: Gibbs energy or Gibbs function; also known as free enthalpy to distinguish it from Helmholtz free energy) is a thermodynamic potential that can be used to calculate the maximum of reversible work that may be performed by a thermodynamic system at a constant temperature and pressure (isothermal, isobaric). The Gibbs free energy (ΔGº = ΔHº-TΔSº) (J in SI units) is the maximum amount of non-expansion work that can be extracted from a thermodynamically closed system (one that can exchange heat and work with its surroundings, but not matter); this maximum can be attained only in a completely reversible process.
When a system transforms reversibly from an initial state to a final
state, the decrease in Gibbs free energy equals the work done by the
system to its surroundings, minus the work of the pressure forces.
The Gibbs energy (also referred to as G) is also the thermodynamic potential that is minimized when a system reaches chemical equilibrium at constant pressure and temperature. Its derivative with respect to the reaction coordinate of the system vanishes at the equilibrium point. As such, a reduction in G is a necessary condition for the spontaneity of processes at constant pressure and temperature.
The Gibbs free energy, originally called available energy, was developed in the 1870s by the American scientist Josiah Willard Gibbs. In 1873, Gibbs described this "available energy" as
The Gibbs energy (also referred to as G) is also the thermodynamic potential that is minimized when a system reaches chemical equilibrium at constant pressure and temperature. Its derivative with respect to the reaction coordinate of the system vanishes at the equilibrium point. As such, a reduction in G is a necessary condition for the spontaneity of processes at constant pressure and temperature.
The Gibbs free energy, originally called available energy, was developed in the 1870s by the American scientist Josiah Willard Gibbs. In 1873, Gibbs described this "available energy" as
the greatest amount of mechanical work which can be obtained from a given quantity of a certain substance in a given initial state, without increasing its total volume or allowing heat to pass to or from external bodies, except such as at the close of the processes are left in their initial condition.The initial state of the body, according to Gibbs, is supposed to be such that "the body can be made to pass from it to states of dissipated energy by reversible processes". In his 1876 magnum opus On the Equilibrium of Heterogeneous Substances, a graphical analysis of multi-phase chemical systems, he engaged his thoughts on chemical free energy in full.
Overview
The reaction C(s)diamond → C(s)graphite
has a negative change in Gibbs free energy and is therefore
thermodynamically favorable at 25 °C and 1 atm. However, even though
favorable, it is so slow that it is not observed. Whether a reaction is
thermodynamically favorable does not determine its rate.
According to the second law of thermodynamics, for systems reacting at STP
(or any other fixed temperature and pressure), there is a general
natural tendency to achieve a minimum of the Gibbs free energy.
A quantitative measure of the favorability of a given reaction at constant temperature and pressure is the change ΔG (sometimes written "delta G" or "dG")
in Gibbs free energy that is (or would be) caused by the reaction. As a
necessary condition for the reaction to occur at constant temperature
and pressure, ΔG must be smaller than the non-PV (e.g. electrical) work, which is often equal to zero (hence ΔG must be negative). ΔG equals the maximum amount of non-PV
work that can be performed as a result of the chemical reaction for the
case of reversible process. If the analysis indicated a positive ΔG for the reaction, then energy — in the form of electrical or other non-PV work — would have to be added to the reacting system for ΔG to be smaller than the non-PV work and make it possible for the reaction to occur.
We can think of ∆G as the amount of "free" or "useful" energy
available to do work. The equation can be also seen from the perspective
of the system taken together with its surroundings (the rest of the
universe). First, assume that the given reaction at constant temperature
and pressure is the only one that is occurring. Then the entropy
released or absorbed by the system equals the entropy that the
environment must absorb or release, respectively. The reaction will only
be allowed if the total entropy change of the universe is zero or
positive. This is reflected in a negative ΔG, and the reaction is called exergonic.
If we couple reactions, then an otherwise endergonic chemical reaction (one with positive ΔG) can be made to happen. The input of heat into an inherently endergonic reaction, such as the elimination of cyclohexanol to cyclohexene,
can be seen as coupling an unfavourable reaction (elimination) to a
favourable one (burning of coal or other provision of heat) such that
the total entropy change of the universe is greater than or equal to
zero, making the total Gibbs free energy difference of the coupled reactions negative.
In traditional use, the term "free" was included in "Gibbs free energy" to mean "available in the form of useful work". The characterization becomes more precise if we add the qualification that it is the energy available for non-volume work.
(An analogous, but slightly different, meaning of "free" applies in
conjunction with the Helmholtz free energy, for systems at constant
temperature). However, an increasing number of books and journal
articles do not include the attachment "free", referring to G as simply "Gibbs energy". This is the result of a 1988 IUPAC
meeting to set unified terminologies for the international scientific
community, in which the adjective "free" was supposedly banished. This standard, however, has not yet been universally adopted.
History
The quantity called "free energy" is a more advanced and accurate replacement for the outdated term affinity, which was used by chemists in the earlier years of physical chemistry to describe the force that caused chemical reactions.
In 1873, Willard Gibbs published A Method of Geometrical Representation of the Thermodynamic Properties of Substances by Means of Surfaces,
in which he sketched the principles of his new equation that was able
to predict or estimate the tendencies of various natural processes to
ensue when bodies or systems are brought into contact. By studying the
interactions of homogeneous substances in contact, i.e., bodies composed
of part solid, part liquid, and part vapor, and by using a
three-dimensional volume-entropy-internal energy
graph, Gibbs was able to determine three states of equilibrium, i.e.,
"necessarily stable", "neutral", and "unstable", and whether or not
changes would ensue. Further, Gibbs stated:
In this description, as used by Gibbs, ε refers to the internal energy of the body, η refers to the entropy of the body, and ν is the volume of the body.
Thereafter, in 1882, the German scientist Hermann von Helmholtz
characterized the affinity as the largest quantity of work which can be
gained when the reaction is carried out in a reversible manner, e.g.,
electrical work in a reversible cell. The maximum work is thus regarded
as the diminution of the free, or available, energy of the system (Gibbs free energy G at T = constant, P = constant or Helmholtz free energy F at T = constant, V = constant), whilst the heat given out is usually a measure of the diminution of the total energy of the system (internal energy). Thus, G or F is the amount of energy "free" for work under the given conditions.
Until this point, the general view had been such that: "all
chemical reactions drive the system to a state of equilibrium in which
the affinities of the reactions vanish". Over the next 60 years, the
term affinity came to be replaced with the term free energy. According
to chemistry historian Henry Leicester, the influential 1923 textbook Thermodynamics and the Free Energy of Chemical Substances by Gilbert N. Lewis and Merle Randall led to the replacement of the term "affinity" by the term "free energy" in much of the English-speaking world.
Graphical interpretation
Gibbs free energy was originally defined graphically. In 1873, American scientist Willard Gibbs
published his first thermodynamics paper, "Graphical Methods in the
Thermodynamics of Fluids", in which Gibbs used the two coordinates of
the entropy and volume to represent the state of the body. In his second
follow-up paper, "A Method of Geometrical Representation of the
Thermodynamic Properties of Substances by Means of Surfaces", published
later that year, Gibbs added in the third coordinate of the energy of
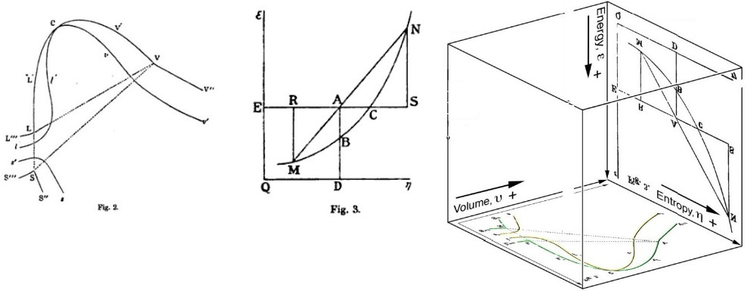
the body, defined on three figures. In 1874, Scottish physicist James Clerk Maxwell used Gibbs' figures to make a 3D energy-entropy-volume thermodynamic surface of a fictitious water-like substance.
Thus, in order to understand the very difficult concept of Gibbs free
energy one must be able to understand its interpretation as Gibbs
defined originally by section AB on his figure 3 and as Maxwell sculpted
that section on his 3D surface figure.
American scientist Willard Gibbs' 1873 figures two and three (above left and middle) used by Scottish physicist James Clerk Maxwell in 1874 to create a three-dimensional entropy, volume, energy thermodynamic surface
diagram for a fictitious water-like substance, transposed the two
figures of Gibbs (above right) onto the volume-entropy coordinates
(transposed to bottom of cube) and energy-entropy coordinates (flipped
upside down and transposed to back of cube), respectively, of a
three-dimensional Cartesian coordinates;
the region AB being the first-ever three-dimensional representation of
Gibbs free energy, or what Gibbs called "available energy"; the region
AC being its capacity for entropy,
what Gibbs defined as "the amount by which the entropy of the body can
be increased without changing the energy of the body or increasing its
volume.
Definitions
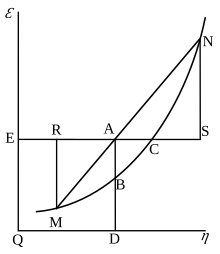
Willard Gibbs’ 1873 available energy (free energy) graph, which shows a plane perpendicular to the axis of v (volume) and passing through point A, which represents the initial state of the body. MN is the section of the surface of dissipated energy. Qε and Qη are sections of the planes η = 0 and ε = 0, and therefore parallel to the axes of ε (internal energy) and η (entropy), respectively. AD and AE are the energy and entropy of the body in its initial state, AB and AC its available energy (Gibbs free energy) and its capacity for entropy
(the amount by which the entropy of the body can be increased without
changing the energy of the body or increasing its volume) respectively.
The Gibbs free energy is defined as
which is the same as
where:
- U is the internal energy (SI unit: joule),
- p is pressure (SI unit: pascal),
- V is volume (SI unit: m3),
- T is the temperature (SI unit: kelvin),
- S is the entropy (SI unit: joule per kelvin),
- H is the enthalpy (SI unit: joule).
The expression for the infinitesimal reversible change in the Gibbs free energy as a function of its "natural variables" p and T, for an open system, subjected to the operation of external forces (for instance, electrical or magnetic) Xi, which cause the external parameters of the system ai to change by an amount dai, can be derived as follows from the first law for reversible processes:
where:
- μi is the chemical potential of the ith chemical component. (SI unit: joules per particle or joules per mole)
- Ni is the number of particles (or number of moles) composing the ith chemical component.
This is one form of Gibbs fundamental equation.
In the infinitesimal expression, the term involving the chemical
potential accounts for changes in Gibbs free energy resulting from an
influx or outflux of particles. In other words, it holds for an open system or for a closed, chemically reacting system where the Ni are changing. For a closed, non-reacting system, this term may be dropped.
Any number of extra terms may be added, depending on the particular system being considered. Aside from mechanical work,
a system may, in addition, perform numerous other types of work. For
example, in the infinitesimal expression, the contractile work energy
associated with a thermodynamic system that is a contractile fiber that
shortens by an amount −dl under a force f would result in a term f dl being added. If a quantity of charge −de is acquired by a system at an electrical potential Ψ, the electrical work associated with this is −Ψ de, which would be included in the infinitesimal expression. Other work terms are added on per system requirements.
Each quantity in the equations above can be divided by the amount of substance, measured in moles, to form molar Gibbs free energy.
The Gibbs free energy is one of the most important thermodynamic
functions for the characterization of a system. It is a factor in
determining outcomes such as the voltage of an electrochemical cell, and the equilibrium constant for a reversible reaction.
In isothermal, isobaric systems, Gibbs free energy can be thought of as
a "dynamic" quantity, in that it is a representative measure of the
competing effects of the enthalpic[clarification needed] and entropic driving forces involved in a thermodynamic process.
Relation to other relevant parameters
The temperature dependence of the Gibbs energy for an ideal gas is given by the Gibbs–Helmholtz equation, and its pressure dependence is given by
If the volume is known rather than pressure, then it becomes
or more conveniently as its chemical potential:
In non-ideal systems, fugacity comes into play.
Derivation
The Gibbs free energy total differential natural variables may be derived by Legendre transforms of the internal energy.
The definition of G from above is
- .
Taking the total differential, we have
Replacing dU with the result from the first law gives
The natural variables of G are then p, T, and {Ni}.
Homogeneous systems
Because S, V, and Ni are extensive variables, an Euler integral allows easy integration of dU:
Because some of the natural variables of G are intensive, dG
may not be integrated using Euler integrals as is the case with
internal energy. However, simply substituting the above integrated
result for U into the definition of G gives a standard expression for G:
This result applies to homogeneous, macroscopic systems, but not to all thermodynamic systems.
Gibbs free energy of reactions
The
system under consideration is held at constant temperature and
pressure, and is closed (no matter can come in or out). The Gibbs energy
of any system is and an infinitesimal change in G, at constant temperature and pressure yields:
By the first law of thermodynamics, a change in the internal energy U is given by
where δQ is energy added as heat, and δW is energy added as work. The work done on the system may be written as δW = −PdV + δWx, where −PdV is the mechanical work of compression/expansion done on the system and δWx is all other forms of work, which may include electrical, magnetic, etc. Assuming that only mechanical work is done,
and the infinitesimal change in G is:
The second law of thermodynamics states that for a closed system, , and so it follows that:
This means that for a system which is not in equilibrium, its Gibbs
energy will always be decreasing, and when it is in equilibrium (i.e. no
longer changing), the infinitesimal change dG will be zero. In
particular, this will be true if the system is experiencing any number
of internal chemical reactions on its path to equilibrium.
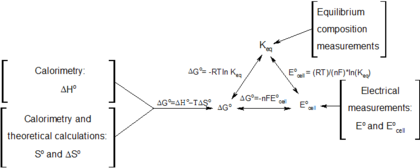
Useful identities to derive the Nernst equation
During a reversible electrochemical reaction at constant temperature
and pressure, the following equations involving the Gibbs free energy
hold:
- ,
- (for a system at chemical equilibrium),
- (for a reversible electrochemical process at constant temperature and pressure),
- (definition of E°),
and rearranging gives
which relates the cell potential resulting from the reaction to the equilibrium constant and reaction quotient for that reaction (Nernst equation),
where
- ΔrG = Gibbs free energy change per mole of reaction,
- ΔrG° = Gibbs free energy change per mole of reaction for unmixed reactants and products at standard conditions (i.e. 298K, 100kPa, 1M of each reactant and product),
- R = gas constant,
- T = absolute temperature,
- ln = natural logarithm,
- Qr = reaction quotient (unitless),
- Keq = equilibrium constant (unitless),
- welec,rev = electrical work in a reversible process (chemistry sign convention),
- n = number of moles of electrons transferred in the reaction,
- F = Faraday constant = 96485 C/mol (charge per mole of electrons),
- E = cell potential,
- E° = standard cell potential.
Moreover, we also have:
which relates the equilibrium constant with Gibbs free energy. This implies that at equilibrium
- and
The second law of thermodynamics and metabolism
A chemical reaction will (or can) proceed spontaneously if the change
in the total entropy of the universe that would be caused by the
reaction is nonnegative. As discussed in the overview, if the
temperature and pressure are held constant, the Gibbs free energy is a
(negative) proxy for the change in total entropy of the universe. It is
"negative" because S appears with a negative coefficient in the expression for G,
so the Gibbs free energy moves in the opposite direction from the total
entropy. Thus, a reaction with a positive Gibbs free energy will not
proceed spontaneously. However, in biological systems (among others),
energy inputs from other energy sources (including the Sun and
exothermic chemical reactions) are "coupled" with reactions that are not
entropically favored (i.e. have a Gibbs free energy above zero). Taking
into account the coupled reactions, the total entropy in the universe
increases. This coupling allows endergonic reactions, such as
photosynthesis and DNA synthesis, to proceed without decreasing the
total entropy of the universe. Thus biological systems do not violate
the second law of thermodynamics.
Standard energy change of formation
| Substance
(State)
|
ΔfG°
(kJ/mol)
|
ΔfG°
(kcal/mol)
|
|---|---|---|
| NO(g) | 87.6 | 20.9 |
| NO2(g) | 51.3 | 12.3 |
| N2O(g) | 103.7 | 24.78 |
| H2O(g) | −228.6 | −54.64 |
| H2O(l) | −237.1 | −56.67 |
| CO2(g) | −394.4 | −94.26 |
| CO(g) | −137.2 | −32.79 |
| CH4(g) | −50.5 | −12.1 |
| C2H6(g) | −32.0 | −7.65 |
| C3H8(g) | −23.4 | −5.59 |
| C6H6(g) | 129.7 | 29.76 |
| C6H6(l) | 124.5 | 31.00 |
The standard Gibbs free energy of formation of a compound is the change of Gibbs free energy that accompanies the formation of 1 mole of that substance from its component elements, at their standard states (the most stable form of the element at 25 °C and 100 kPa). Its symbol is ΔfG˚.
All elements in their standard states (diatomic oxygen gas, graphite, etc.) have standard Gibbs free energy change of formation equal to zero, as there is no change involved.
- ΔfG = ΔfG˚ + RT ln Qf ;
- Qf is the reaction quotient.
- At equilibrium, ΔfG = 0, and Qf = K, so the equation becomes ΔfG˚ = −RT ln K (where K is the equilibrium constant).