Cell-count distribution featuring cellular differentiation for three types of cells (progenitor , osteoblast , and chondrocyte ) exposed to pro-osteoblast stimulus.
Cellular differentiation is the process where a cell changes from one cell type to another. Usually, the cell changes to a more specialized type. Differentiation occurs numerous times during the development of a multicellular organism as it changes from a simple zygote to a complex system of tissues and cell types. Differentiation continues in adulthood as adult stem cells divide and create fully differentiated daughter cells during tissue repair and during normal cell turnover. Some differentiation occurs in response to antigen exposure. Differentiation dramatically changes a cell's size, shape, membrane potential, metabolic activity, and responsiveness to signals. These changes are largely due to highly controlled modifications in gene expression and are the study of epigenetics. With a few exceptions, cellular differentiation almost never involves a change in the DNA sequence itself. Thus, different cells can have very different physical characteristics despite having the same genome.
A specialized type of differentiation, known as 'terminal
differentiation', is of importance in some tissues, for example
vertebrate nervous system, striated muscle, epidermis and gut. During
terminal differentiation, a precursor cell formerly capable of cell
division, permanently leaves the cell cycle, dismantles the cell cycle
machinery and often expresses a range of genes characteristic of the
cell's final function (e.g. myosin and actin for a muscle cell).
Differentiation may continue to occur after terminal differentiation if
the capacity and functions of the cell undergo further changes.
Among dividing cells, there are multiple levels of cell potency,
the cell's ability to differentiate into other cell types. A greater
potency indicates a larger number of cell types that can be derived. A
cell that can differentiate into all cell types, including the placental
tissue, is known as totipotent. In mammals, only the zygote and subsequent blastomeres
are totipotent, while in plants, many differentiated cells can become
totipotent with simple laboratory techniques. A cell that can
differentiate into all cell types of the adult organism is known as pluripotent. Such cells are called meristematic cells in higher plants and embryonic stem cells
in animals, though some groups report the presence of adult pluripotent
cells. Virally induced expression of four transcription factors Oct4, Sox2, c-Myc, and KIF4 (Yamanaka factors) is sufficient to create pluripotent (iPS) cells from adult fibroblasts. A multipotent cell is one that can differentiate into multiple different, but closely related cell types. Oligopotent cells are more restricted than multipotent, but can still differentiate into a few closely related cell types. Finally, unipotent cells can differentiate into only one cell type, but are capable of self-renewal. In cytopathology, the level of cellular differentiation is used as a measure of cancer progression. "Grade" is a marker of how differentiated a cell in a tumor is.
Mammalian cell types
Three basic categories of cells make up the mammalian body: germ cells, somatic cells, and stem cells. Each of the approximately 37.2 trillion (3.72x1013) cells in an adult human has its own copy or copies of the genome except certain cell types, such as red blood cells, that lack nuclei in their fully differentiated state. Most cells are diploid; they have two copies of each chromosome.
Such cells, called somatic cells, make up most of the human body, such
as skin and muscle cells. Cells differentiate to specialize for
different functions.
Germ line cells are any line of cells that give rise to gametes—eggs
and sperm—and thus are continuous through the generations. Stem cells,
on the other hand, have the ability to divide for indefinite periods and
to give rise to specialized cells. They are best described in the
context of normal human development.
Development begins when a sperm fertilizes an egg
and creates a single cell that has the potential to form an entire
organism. In the first hours after fertilization, this cell divides into
identical cells. In humans, approximately four days after fertilization
and after several cycles of cell division, these cells begin to
specialize, forming a hollow sphere of cells, called a blastocyst. The blastocyst has an outer layer of cells, and inside this hollow sphere, there is a cluster of cells called the inner cell mass.
The cells of the inner cell mass go on to form virtually all of the
tissues of the human body. Although the cells of the inner cell mass can
form virtually every type of cell found in the human body, they cannot
form an organism. These cells are referred to as pluripotent.
Pluripotent stem cells undergo further specialization into multipotent progenitor cells that then give rise to functional cells. Examples of stem and progenitor cells include:
- Radial glial cells (embryonic neural stem cells) that give rise to excitatory neurons in the fetal brain through the process of neurogenesis.
- Hematopoietic stem cells (adult stem cells) from the bone marrow that give rise to red blood cells, white blood cells, and platelets
- Mesenchymal stem cells (adult stem cells) from the bone marrow that give rise to stromal cells, fat cells, and types of bone cells
- Epithelial stem cells (progenitor cells) that give rise to the various types of skin cells
- Muscle satellite cells (progenitor cells) that contribute to differentiated muscle tissue.
A pathway that is guided by the cell adhesion molecules consisting of four amino acids, arginine, glycine, asparagine, and serine, is created as the cellular blastomere differentiates from the single-layered blastula to the three primary layers of germ cells in mammals, namely the ectoderm, mesoderm and endoderm
(listed from most distal (exterior) to proximal (interior)). The
ectoderm ends up forming the skin and the nervous system, the mesoderm
forms the bones and muscular tissue, and the endoderm forms the internal
organ tissues.
Dedifferentiation
Micrograph of a liposarcoma
with some dedifferentiation, that is not identifiable as a liposarcoma,
(left edge of image) and a differentiated component (with lipoblasts and increased vascularity (right of image)). Fully differentiated (morphologically benign) adipose tissue (center of the image) has few blood vessels. H&E stain.
Dedifferentiation, or integration is a cellular process often seen in more basal life forms such as worms and amphibians in which a partially or terminally differentiated cell reverts to an earlier developmental stage, usually as part of a regenerative process. Dedifferentiation also occurs in plants. Cells in cell culture
can lose properties they originally had, such as protein expression, or
change shape. This process is also termed dedifferentiation.
Some believe dedifferentiation is an aberration of the normal development cycle that results in cancer, whereas others believe it to be a natural part of the immune response lost by humans at some point as a result of evolution.
A small molecule dubbed reversine, a purine analog, has been discovered that has proven to induce dedifferentiation in myotubes. These dedifferentiated cells could then redifferentiate into osteoblasts and adipocytes.
Diagram exposing several methods used to revert adult somatic cells to totipotency or pluripotency.
Mechanisms
Mechanisms of cellular differentiation.
Each specialized cell type in an organism expresses a subset of all the genes that constitute the genome of that species. Each cell type is defined by its particular pattern of regulated gene expression.
Cell differentiation is thus a transition of a cell from one cell type
to another and it involves a switch from one pattern of gene expression
to another. Cellular differentiation during development can be
understood as the result of a gene regulatory network.
A regulatory gene and its cis-regulatory modules are nodes in a gene
regulatory network; they receive input and create output elsewhere in
the network. The systems biology
approach to developmental biology emphasizes the importance of
investigating how developmental mechanisms interact to produce
predictable patterns (morphogenesis). (However, an alternative view has been proposed recently. Based on stochastic
gene expression, cellular differentiation is the result of a Darwinian
selective process occurring among cells. In this frame, protein and gene
networks are the result of cellular processes and not their cause.)
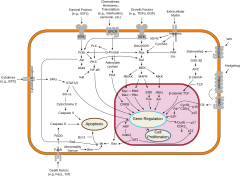
An overview of major signal transduction pathways.
A few evolutionarily
conserved types of molecular processes are often involved in the
cellular mechanisms that control these switches. The major types of
molecular processes that control cellular differentiation involve cell signaling.
Many of the signal molecules that convey information from cell to cell
during the control of cellular differentiation are called growth factors. Although the details of specific signal transduction
pathways vary, these pathways often share the following general steps.
A ligand produced by one cell binds to a receptor in the extracellular
region of another cell, inducing a conformational change in the
receptor. The shape of the cytoplasmic domain of the receptor changes,
and the receptor acquires enzymatic activity. The receptor then
catalyzes reactions that phosphorylate other proteins, activating them.
A cascade of phosphorylation reactions eventually activates a dormant
transcription factor or cytoskeletal protein, thus contributing to the
differentiation process in the target cell. Cells and tissues can vary in competence, their ability to respond to external signals.
Signal induction refers to cascades of signaling events, during which a cell or tissue signals to another cell or tissue to influence its developmental fate. Yamamoto and Jeffery investigated the role of the lens in eye formation in cave- and surface-dwelling fish, a striking example of induction. Through reciprocal transplants, Yamamoto and Jeffery
found that the lens vesicle of surface fish can induce other parts of
the eye to develop in cave- and surface-dwelling fish, while the lens
vesicle of the cave-dwelling fish cannot.
Other important mechanisms fall under the category of asymmetric cell divisions,
divisions that give rise to daughter cells with distinct developmental
fates. Asymmetric cell divisions can occur because of asymmetrically
expressed maternal cytoplasmic determinants or because of signaling. In the former mechanism, distinct daughter cells are created during cytokinesis
because of an uneven distribution of regulatory molecules in the parent
cell; the distinct cytoplasm that each daughter cell inherits results
in a distinct pattern of differentiation for each daughter cell. A
well-studied example of pattern formation by asymmetric divisions is body axis patterning in Drosophila. RNA
molecules are an important type of intracellular differentiation
control signal. The molecular and genetic basis of asymmetric cell
divisions has also been studied in green algae of the genus Volvox, a model system for studying how unicellular organisms can evolve into multicellular organisms. In Volvox carteri,
the 16 cells in the anterior hemisphere of a 32-cell embryo divide
asymmetrically, each producing one large and one small daughter cell.
The size of the cell at the end of all cell divisions determines whether
it becomes a specialized germ or somatic cell.
Epigenetic control
Since each cell, regardless of cell type, possesses the same genome, determination of cell type must occur at the level of gene expression. While the regulation of gene expression can occur through cis- and trans-regulatory elements including a gene's promoter and enhancers, the problem arises as to how this expression pattern is maintained over numerous generations of cell division. As it turns out, epigenetic
processes play a crucial role in regulating the decision to adopt a
stem, progenitor, or mature cell fate. This section will focus primarily
on mammalian stem cells.
In systems biology and mathematical modeling of gene regulatory
networks, cell-fate determination is predicted to exhibit certain
dynamics, such as attractor-convergence (the attractor can be an
equilibrium point, limit cycle or strange attractor) or oscillatory.
Importance of epigenetic control
The
first question that can be asked is the extent and complexity of the
role of epigenetic processes in the determination of cell fate. A clear
answer to this question can be seen in the 2011 paper by Lister R, et al. on aberrant epigenomic programming in human induced pluripotent stem cells. As induced pluripotent stem cells (iPSCs) are thought to mimic embryonic stem cells
in their pluripotent properties, few epigenetic differences should
exist between them. To test this prediction, the authors conducted
whole-genome profiling of DNA methylation patterns in several human embryonic stem cell (ESC), iPSC, and progenitor cell lines.
Female adipose cells, lung fibroblasts, and foreskin fibroblasts were reprogrammed into induced pluripotent state with the OCT4, SOX2, KLF4, and MYC genes. Patterns of DNA methylation in ESCs, iPSCs, somatic cells were compared. Lister R, et al. observed significant resemblance in methylation levels between embryonic and induced pluripotent cells. Around 80% of CG dinucleotides
in ESCs and iPSCs were methylated, the same was true of only 60% of CG
dinucleotides in somatic cells. In addition, somatic cells possessed
minimal levels of cytosine methylation in non-CG dinucleotides, while
induced pluripotent cells possessed similar levels of methylation as
embryonic stem cells, between 0.5 and 1.5%. Thus, consistent with their
respective transcriptional activities, DNA methylation patterns, at least on the genomic level, are similar between ESCs and iPSCs.
However, upon examining methylation patterns more closely, the
authors discovered 1175 regions of differential CG dinucleotide
methylation between at least one ES or iPS cell line. By comparing these
regions of differential methylation with regions of cytosine
methylation in the original somatic cells, 44-49% of differentially
methylated regions reflected methylation patterns of the respective
progenitor somatic cells, while 51-56% of these regions were dissimilar
to both the progenitor and embryonic cell lines. In vitro-induced
differentiation of iPSC lines saw transmission of 88% and 46% of hyper
and hypo-methylated differentially methylated regions, respectively.
Two conclusions are readily apparent from this study. First,
epigenetic processes are heavily involved in cell fate determination, as
seen from the similar levels of cytosine methylation between induced
pluripotent and embryonic stem cells, consistent with their respective
patterns of transcription.
Second, the mechanisms of de-differentiation (and by extension,
differentiation) are very complex and cannot be easily duplicated, as
seen by the significant number of differentially methylated regions
between ES and iPS cell lines. Now that these two points have been
established, we can examine some of the epigenetic mechanisms that are
thought to regulate cellular differentiation.
Mechanisms of epigenetic regulation
Pioneer factor|Pioneering factors (Oct4, Sox2, Nanog)
Three transcription factors, OCT4, SOX2, and NANOG – the first two of which are used in induced pluripotent stem cell (iPSC) reprogramming, along with Klf4 and c-Myc – are highly expressed in undifferentiated embryonic stem cells and are necessary for the maintenance of their pluripotency. It is thought that they achieve this through alterations in chromatin structure, such as histone modification
and DNA methylation, to restrict or permit the transcription of target
genes. While highly expressed, their levels require a precise balance to
maintain pluripotency, perturbation of which will promote
differentiation towards different lineages based on how the gene
expression levels change. Differential regulation of Oct-4 and SOX2 levels have been shown to precede germ layer fate selection. Increased levels of Oct4 and decreased levels of Sox2 promote a mesendodermal fate, with Oct4 actively suppressing genes associated with a neural ectodermal
fate. Similarly, Increased levels of Sox2 and decreased levels of Oct4
promote differentiation towards a neural ectodermal fate, with Sox2
inhibiting differentiation towards a mesendodermal fate. Regardless of
the lineage cells differentiate down, suppression of NANOG has been identified as a necessary prerequisite for differentiation.
Polycomb repressive complex (PRC2)
In the realm of gene silencing, Polycomb repressive complex 2, one of two classes of the Polycomb group (PcG) family of proteins, catalyzes the di- and tri-methylation of histone H3 lysine 27 (H3K27me2/me3).
By binding to the H3K27me2/3-tagged nucleosome, PRC1 (also a complex of
PcG family proteins) catalyzes the mono-ubiquitinylation of histone H2A
at lysine 119 (H2AK119Ub1), blocking RNA polymerase II activity and resulting in transcriptional suppression.
PcG knockout ES cells do not differentiate efficiently into the three
germ layers, and deletion of the PRC1 and PRC2 genes leads to increased
expression of lineage-affiliated genes and unscheduled differentiation. Presumably, PcG complexes are responsible for transcriptionally repressing differentiation and development-promoting genes.
Trithorax group proteins (TrxG)
Alternately,
upon receiving differentiation signals, PcG proteins are recruited to
promoters of pluripotency transcription factors. PcG-deficient ES cells
can begin differentiation but cannot maintain the differentiated
phenotype.
Simultaneously, differentiation and development-promoting genes are
activated by Trithorax group (TrxG) chromatin regulators and lose their
repression.
TrxG proteins are recruited at regions of high transcriptional
activity, where they catalyze the trimethylation of histone H3 lysine 4 (H3K4me3) and promote gene activation through histone acetylation.
PcG and TrxG complexes engage in direct competition and are thought to
be functionally antagonistic, creating at differentiation and
development-promoting loci what is termed a "bivalent domain" and
rendering these genes sensitive to rapid induction or repression.
DNA methylation
Regulation of gene expression is further achieved through DNA methylation, in which the DNA methyltransferase-mediated methylation of cytosine residues in CpG dinucleotides maintains heritable repression by controlling DNA accessibility.
The majority of CpG sites in embryonic stem cells are unmethylated and
appear to be associated with H3K4me3-carrying nucleosomes. Upon differentiation, a small number of genes, including OCT4 and NANOG,
are methylated and their promoters repressed to prevent their further
expression. Consistently, DNA methylation-deficient embryonic stem cells
rapidly enter apoptosis upon in vitro differentiation.
Nucleosome positioning
While the DNA sequence
of most cells of an organism is the same, the binding patterns of
transcription factors and the corresponding gene expression patterns are
different. To a large extent, differences in transcription factor
binding are determined by the chromatin accessibility of their binding
sites through histone modification and/or pioneer factors. In particular, it is important to know whether a nucleosome is covering a given genomic binding site or not. This can be determined using a chromatin immunoprecipitation (ChIP) assay.
Histone acetylation and methylation
DNA-nucleosome
interactions are characterized by two states: either tightly bound by
nucleosomes and transcriptionally inactive, called heterochromatin, or loosely bound and usually, but not always, transcriptionally active, called euchromatin.
The epigenetic processes of histone methylation and acetylation, and
their inverses demethylation and deacetylation primarily account for
these changes. The effects of acetylation and deacetylation are more
predictable. An acetyl group is either added to or removed from the
positively charged Lysine residues in histones by enzymes called histone acetyltransferases or histone deacteylases,
respectively. The acetyl group prevents Lysine's association with the
negatively charged DNA backbone. Methylation is not as straightforward,
as neither methylation nor demethylation consistently correlate with
either gene activation or repression. However, certain methylations have
been repeatedly shown to either activate or repress genes. The
trimethylation of lysine 4 on histone 3 (H3K4Me3) is associated with
gene activation, whereas trimethylation of lysine 27 on histone 3
represses genes.
In stem cells
During differentiation, stem cells
change their gene expression profiles. Recent studies have implicated a
role for nucleosome positioning and histone modifications during this
process.
There are two components of this process: turning off the expression of
embryonic stem cell (ESC) genes, and the activation of cell fate genes.
Lysine specific demethylase 1 (KDM1A) is thought to prevent the use of enhancer regions of pluripotency genes, thereby inhibiting their transcription. It interacts with Mi-2/NuRD complex (nucleosome remodelling and histone deacetylase) complex, giving an instance where methylation and acetylation are not discrete and mutually exclusive, but intertwined processes.
Role of signaling in epigenetic control
A final question to ask concerns the role of cell signaling
in influencing the epigenetic processes governing differentiation. Such
a role should exist, as it would be reasonable to think that extrinsic
signaling can lead to epigenetic remodeling, just as it can lead to
changes in gene expression through the activation or repression of
different transcription factors. Little direct data is available
concerning the specific signals that influence the epigenome,
and the majority of current knowledge about the subject consists of
speculations on plausible candidate regulators of epigenetic remodeling.
We will first discuss several major candidates thought to be involved
in the induction and maintenance of both embryonic stem cells and their
differentiated progeny, and then turn to one example of specific
signaling pathways in which more direct evidence exists for its role in
epigenetic change.
The first major candidate is Wnt signaling pathway.
The Wnt pathway is involved in all stages of differentiation, and the
ligand Wnt3a can substitute for the overexpression of c-Myc in the
generation of induced pluripotent stem cells. On the other hand, disruption of ß-catenin, a component of the Wnt signaling pathway, leads to decreased proliferation of neural progenitors.
Growth factors
comprise the second major set of candidates of epigenetic regulators of
cellular differentiation. These morphogens are crucial for development,
and include bone morphogenetic proteins, transforming growth factors (TGFs), and fibroblast growth factors (FGFs). TGFs and FGFs have been shown to sustain expression of OCT4, SOX2, and NANOG by downstream signaling to Smad proteins.
Depletion of growth factors promotes the differentiation of ESCs, while
genes with bivalent chromatin can become either more restrictive or
permissive in their transcription.
Several other signaling pathways are also considered to be primary candidates. Cytokine leukemia inhibitory factors
are associated with the maintenance of mouse ESCs in an
undifferentiated state. This is achieved through its activation of the
Jak-STAT3 pathway, which has been shown to be necessary and sufficient
towards maintaining mouse ESC pluripotency. Retinoic acid can induce differentiation of human and mouse ESCs, and Notch signaling is involved in the proliferation and self-renewal of stem cells. Finally, Sonic hedgehog,
in addition to its role as a morphogen, promotes embryonic stem cell
differentiation and the self-renewal of somatic stem cells.
The problem, of course, is that the candidacy of these signaling
pathways was inferred primarily on the basis of their role in
development and cellular differentiation. While epigenetic regulation is
necessary for driving cellular differentiation, they are certainly not
sufficient for this process. Direct modulation of gene expression
through modification of transcription factors plays a key role that must
be distinguished from heritable epigenetic changes that can persist
even in the absence of the original environmental signals. Only a few
examples of signaling pathways leading to epigenetic changes that alter
cell fate currently exist, and we will focus on one of them.
Expression of Shh (Sonic hedgehog) upregulates the production of BMI1, a component of the PcG complex that recognizes H3K27me3. This occurs in a Gli-dependent manner, as Gli1 and Gli2 are downstream effectors of the Hedgehog signaling pathway. In culture, Bmi1 mediates the Hedgehog pathway's ability to promote human mammary stem cell self-renewal.
In both humans and mice, researchers showed Bmi1 to be highly expressed
in proliferating immature cerebellar granule cell precursors. When Bmi1
was knocked out in mice, impaired cerebellar development resulted,
leading to significant reductions in postnatal brain mass along with
abnormalities in motor control and behavior.
A separate study showed a significant decrease in neural stem cell
proliferation along with increased astrocyte proliferation in Bmi null
mice.
A alternative model of cellular differentiation during
embryogenesis is that positional information is based on mechanical
signalling by the cytoskeleton using Embryonic differentiation waves.
The mechanical signal is then epigenetically transduced via signal
transduction systems (of which specific molecules such as Wnt are part)
to result in differential gene expression.
In summary, the role of signaling in the epigenetic control of
cell fate in mammals is largely unknown, but distinct examples exist
that indicate the likely existence of further such mechanisms.
Effect of matrix elasticity
In
order to fulfill the purpose of regenerating a variety of tissues,
adult stems are known to migrate from their niches, adhere to new
extracellular matrices (ECM) and differentiate. The ductility of these
microenvironments are unique to different tissue types. The ECM
surrounding brain, muscle and bone tissues range from soft to stiff. The
transduction of the stem cells into these cells types is not directed
solely by chemokine cues and cell to cell signaling. The elasticity of
the microenvironment can also affect the differentiation of mesenchymal
stem cells (MSCs which originate in bone marrow.) When MSCs are placed
on substrates of the same stiffness as brain, muscle and bone ECM, the
MSCs take on properties of those respective cell types.
Matrix sensing requires the cell to pull against the matrix at focal
adhesions, which triggers a cellular mechano-transducer to generate a
signal to be informed what force is needed to deform the matrix. To
determine the key players in matrix-elasticity-driven lineage
specification in MSCs, different matrix microenvironments were mimicked.
From these experiments, it was concluded that focal adhesions of the
MSCs were the cellular mechano-transducer sensing the differences of the
matrix elasticity. The non-muscle myosin IIa-c isoforms generates the
forces in the cell that lead to signaling of early commitment markers.
Nonmuscle myosin IIa generates the least force increasing to non-muscle
myosin IIc. There are also factors in the cell that inhibit non-muscle
myosin II, such as blebbistatin. This makes the cell effectively blind
to the surrounding matrix.
Researchers have obtained some success in inducing stem cell-like
properties in HEK 239 cells by providing a soft matrix without the use
of diffusing factors.
The stem-cell properties appear to be linked to tension in the cells'
actin network. One identified mechanism for matrix-induced
differentiation is tension-induced proteins, which remodel chromatin in
response to mechanical stretch. The RhoA pathway is also implicated in this process.