| DNA-directed DNA polymerase | |
|---|---|

3D structure of the DNA-binding helix-turn-helix motifs in human DNA polymerase beta (based on PDB file 7ICG)
| |
| Identifiers | |
| EC number | 2.7.7.7 |
| CAS number | 9012-90-2 |
| Databases | |
| IntEnz | IntEnz view |
| BRENDA | BRENDA entry |
| ExPASy | NiceZyme view |
| KEGG | KEGG entry |
| MetaCyc | metabolic pathway |
| PRIAM | profile |
| PDB structures | RCSB PDB PDBe PDBsum |
| Gene Ontology | AmiGO / QuickGO |
DNA polymerase is an enzyme that synthesizes DNA molecules from deoxyribonucleotides, the building blocks of DNA. These enzymes are essential for DNA replication
and usually work in pairs to create two identical DNA strands from a
single original DNA molecule. During this process, DNA polymerase
"reads" the existing DNA strands to create two new strands that match
the existing ones.
These enzymes catalyze the following chemical reaction
- deoxynucleoside triphosphate + DNAn ⇌ diphosphate + DNAn+1
DNA polymerase adds nucleotides to the three prime (3')-end of a DNA strand, one nucleotide at a time.
Every time a cell divides,
DNA polymerases are required to help duplicate the cell's DNA, so that a
copy of the original DNA molecule can be passed to each daughter cell.
In this way, genetic information is passed down from generation to
generation.
Before replication can take place, an enzyme called helicase unwinds the DNA molecule from its tightly woven form, in the process breaking the hydrogen bonds between the nucleotide bases.
This opens up or "unzips" the double-stranded DNA to give two single
strands of DNA that can be used as templates for replication.
History
In 1956, Arthur Kornberg and colleagues discovered DNA polymerase I (Pol I), in Escherichia coli.
They described the DNA replication process by which DNA polymerase
copies the base sequence of a template DNA strand. Kornberg was later
awarded the Nobel Prize in Physiology or Medicine in 1959 for this work. DNA polymerase II was also discovered by Thomas Kornberg (the son of Arthur Kornberg) and Malcolm E. Gefter in 1970 while further elucidating the role of Pol I in E. coli DNA replication.
Function
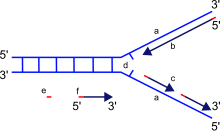
DNA polymerase moves along the old strand in the 3'–5' direction, creating a new strand having a 5'–3' direction.
DNA polymerase with proofreading ability
The main function of DNA polymerase is to synthesize DNA from deoxyribonucleotides,
the building blocks of DNA. The DNA copies are created by the pairing
of nucleotides to bases present on each strand of the original DNA
molecule. This pairing always occurs in specific combinations, with cytosine along with guanine, and thymine along with adenine, forming two separate pairs, respectively. By contrast, RNA polymerases synthesize RNA from ribonucleotides from either RNA or DNA.
When synthesizing new DNA, DNA polymerase can add free nucleotides only to the 3' end
of the newly forming strand. This results in elongation of the newly
forming strand in a 5'–3' direction. No known DNA polymerase is able to
begin a new chain (de novo); it can only add a nucleotide onto a pre-existing 3'-OH group, and therefore needs a primer at which it can add the first nucleotide. Primers consist of RNA or DNA bases (or both). In DNA replication, the first two bases are always RNA, and are synthesized by another enzyme called primase. Helicase and topoisomerase II
are required to unwind DNA from a double-strand structure to a
single-strand structure to facilitate replication of each strand
consistent with the semiconservative model of DNA replication.
It is important to note that the directionality of the newly
forming strand (the daughter strand) is opposite to the direction in
which DNA polymerase moves along the template strand. Since DNA
polymerase requires a free 3' OH group for initiation of synthesis, it
can synthesize in only one direction by extending the 3' end of the
preexisting nucleotide chain. Hence, DNA polymerase moves along the
template strand in a 3'–5' direction, and the daughter strand is formed
in a 5'–3' direction. This difference enables the resultant
double-strand DNA formed to be composed of two DNA strands that are antiparallel to each other.
The function of DNA polymerase is not quite perfect, with the
enzyme making about one mistake for every billion base pairs copied.
Error correction is a property of some, but not all DNA polymerases.
This process corrects mistakes in newly synthesized DNA. When an
incorrect base pair is recognized, DNA polymerase moves backwards by one
base pair of DNA. The 3'–5' exonuclease activity of the enzyme allows the incorrect base pair to be excised (this activity is known as proofreading).
Following base excision, the polymerase can re-insert the correct base
and replication can continue forwards. This preserves the integrity of
the original DNA strand that is passed onto the daughter cells.
Fidelity is very important in DNA replication. Mismatches in DNA base pairing can potentially result in dysfunctional proteins
and could lead to cancer. Many DNA polymerases contain an exonuclease
domain, which acts in detecting base pair mismatches and further
performs in the removal of the incorrect nucleotide to be replaced by
the correct one.
The shape and the interactions accommodating the Watson and Crick base
pair are what primarily contribute to the detection or error. Hydrogen
bonds play a key role in base pair binding and interaction. The loss of
an interaction, which occurs at a mismatch, is said to trigger a shift
in the balance, for the binding of the template-primer, from the
polymerase, to the exonuclease domain. In addition, an incorporation of a
wrong nucleotide causes a retard in DNA polymerization. This delay
gives time for the DNA to be switched from the polymerase site to the
exonuclease site. Different conformational changes and loss of
interaction occur at different mismatches. In a purine:pyrimidine
mismatch there is a displacement of the pyrimidine towards the major
groove and the purine towards the minor groove. Relative to the shape of
DNA polymerase's binding pocket, steric clashes occur between the
purine and residues in the minor groove, and important van der Waals and electrostatic interactions are lost by the pyrimidine.
Pyrimidine:pyrimidine and purine:purine mismatches present less notable
changes since the bases are displaced towards the major groove, and
less steric hindrance is experienced. However, although the different
mismatches result in different steric properties, DNA polymerase is
still able to detect and differentiate them so uniformly and maintain
fidelity in DNA replication. DNA polymerization is also critical for many mutagenesis processes and is widely employed in biotechnologies.
Structure
The known DNA polymerases have highly conserved structure, which means that their overall catalytic subunits
vary very little from species to species, independent of their domain
structures. Conserved structures usually indicate important,
irreplaceable functions of the cell, the maintenance of which provides
evolutionary advantages. The shape can be described as resembling a
right hand with thumb, finger, and palm domains. The palm domain appears
to function in catalyzing the transfer of phosphoryl groups
in the phosphoryl transfer reaction. DNA is bound to the palm when the
enzyme is active. This reaction is believed to be catalyzed by a
two-metal-ion mechanism. The finger domain functions to bind the nucleoside triphosphates
with the template base. The thumb domain plays a potential role in the
processivity, translocation, and positioning of the DNA.
Processivity
DNA polymerase's rapid catalysis is due to its processive nature. Processivity
is a characteristic of enzymes that function on polymeric substrates.
In the case of DNA polymerase, the degree of processivity refers to the
average number of nucleotides added each time the enzyme binds a
template. The average DNA polymerase requires about one second locating
and binding a primer/template junction. Once it is bound, a
nonprocessive DNA polymerase adds nucleotides at a rate of one nucleotide per second.
Processive DNA polymerases, however, add multiple nucleotides per
second, drastically increasing the rate of DNA synthesis. The degree of
processivity is directly proportional to the rate of DNA synthesis. The
rate of DNA synthesis in a living cell was first determined as the rate
of phage T4 DNA elongation in phage infected E. coli. During the period of exponential DNA increase at 37 °C, the rate was 749 nucleotides per second.
DNA polymerase's ability to slide along the DNA template allows
increased processivity. There is a dramatic increase in processivity at
the replication fork. This increase is facilitated by the DNA polymerase's association with proteins known as the sliding DNA clamp. The clamps are multiple protein subunits associated in the shape of a ring. Using the hydrolysis of ATP, a class of proteins known as the sliding clamp loading proteins open up the ring structure of the sliding DNA clamps allowing binding to and release from the DNA strand. Protein-protein interaction
with the clamp prevents DNA polymerase from diffusing from the DNA
template, thereby ensuring that the enzyme binds the same
primer/template junction and continues replication.
DNA polymerase changes conformation, increasing affinity to the clamp
when associated with it and decreasing affinity when it completes the
replication of a stretch of DNA to allow release from the clamp.
Variation across species
| DNA polymerase family A | |
|---|---|

c:o6-methyl-guanine pair in the polymerase-2 basepair position
| |
| Identifiers | |
| Symbol | DNA_pol_A |
| Pfam | PF00476 |
| InterPro | IPR001098 |
| SMART | - |
| PROSITE | PDOC00412 |
| SCOP | 1dpi |
| SUPERFAMILY | 1dpi |
| DNA polymerase family B | |
|---|---|

crystal structure of rb69 gp43 in complex with dna containing thymine glycol
| |
| Identifiers | |
| Symbol | DNA_pol_B |
| Pfam | PF00136 |
| Pfam clan | CL0194 |
| InterPro | IPR006134 |
| PROSITE | PDOC00107 |
| SCOP | 1noy |
| SUPERFAMILY | 1noy |
| DNA polymerase type B, organellar and viral | |
|---|---|

phi29 dna polymerase, orthorhombic crystal form, ssdna complex
| |
| Identifiers | |
| Symbol | DNA_pol_B_2 |
| Pfam | PF03175 |
| Pfam clan | CL0194 |
| InterPro | IPR004868 |
Based on sequence homology, DNA polymerases can be further subdivided into seven different families: A, B, C, D, X, Y, and RT.
Some viruses also encode special DNA polymerases, such as Hepatitis B virus DNA polymerase. These may selectively replicate viral DNA through a variety of mechanisms. Retroviruses encode an unusual DNA polymerase called reverse transcriptase, which is an RNA-dependent DNA polymerase (RdDp). It polymerizes DNA from a template of RNA.
| Family | Types of DNA polymerase | Species | Examples | Feature |
|---|---|---|---|---|
| A | Replicative and Repair Polymerases | Eukaryotic and Prokaryotic | T7 DNA polymerase, Pol I, Pol γ, θ, and ν | Two exonuclease domains (3'-5' and 5'-3') |
| B | Replicative and Repair Polymerases | Eukaryotic and Prokaryotic | Pol II, Pol B, Pol ζ, Pol α, δ, and ε | 3'-5 exonuclease (proofreading); viral ones use protein primer |
| C | Replicative Polymerases | Prokaryotic | Pol III | 3'-5 exonuclease (proofreading) |
| D | Replicative Polymerases | Euryarchaeota | PolD (DP1/DP2 heterodimer) | No "hand" feature, RNA polymerase-like; 3'-5 exonuclease (proofreading) |
| X | Replicative and Repair Polymerases | Eukaryotic | Pol β, Pol σ, Pol λ, Pol μ, and Terminal deoxynucleotidyl transferase | template-independent; 5' phosphatase (only Pol β) |
| Y | Replicative and Repair Polymerases | Eukaryotic and Prokaryotic | Pol ι, Pol κ, Pol η, Pol IV, and Pol V |
|
| RT | Replicative and Repair Polymerases | Viruses, Retroviruses, and Eukaryotic | Telomerase, Hepatitis B virus | RNA-dependent |
Prokaryotic polymerase
Prokaryotes
only have one DNA polymerase and it exists in two forms: core
polymerase and holoenzyme. Core polymerase synthesizes DNA from the DNA
template but it cannot initiate the synthesis alone or accurately.
Holoenzyme accurately initiates synthesis.
Pol I
Prokaryotic family A polymerases include the DNA polymerase I (Pol I) enzyme, which is encoded by the polA gene and ubiquitous among prokaryotes. This repair polymerase is involved in excision repair with both 3'–5' and 5'–3' exonuclease activity and processing of Okazaki fragments generated during lagging strand synthesis. Pol I is the most abundant polymerase, accounting for more than 95% of polymerase activity in E. coli;
yet cells lacking Pol I have been found suggesting Pol I activity can
be replaced by the other four polymerases. Pol I adds ~15-20
nucleotides per second, thus showing poor processivity. Instead, Pol I
starts adding nucleotides at the RNA primer:template junction known as
the origin of replication
(ori). Approximately 400 bp downstream from the origin, the Pol III
holoenzyme is assembled and takes over replication at a highly
processive speed and nature.
Taq polymerase is a heat-stable enzyme of this family that lacks proofreading ability.
Pol II
DNA polymerase II, a family B polymerase, is a polB gene product also known as DnaA. Pol II has 3'–5' exonuclease activity and participates in DNA repair,
replication restart to bypass lesions, and its cell presence can jump
from ~30-50 copies per cell to ~200–300 during SOS induction. Pol II is
also thought to be a backup to Pol III as it can interact with
holoenzyme proteins and assume a high level of processivity. The main
role of Pol II is thought to be the ability to direct polymerase
activity at the replication fork and helped stalled Pol III bypass
terminal mismatches.
Pfu DNA polymerase is a heat-stable enzyme of this family found in the hyperthermophilic archaeon Pyrococcus furiosus. Detailed classification divides family B in archaea into B1, B2, B3, in which B2 is a group of pseudoenzymes. Pfu belongs to family B3. Others PolBs found in archaea are part of "Casposons", Cas1-dependent transposons. Some viruses (including Φ29 DNA polymerase) and mitochondrial plasmids carry polB as well.
Pol III
DNA polymerase III holoenzyme is the primary enzyme involved in DNA replication in E. coli and belongs to family C polymerases. It consists of three assemblies: the pol III core, the beta sliding clamp
processivity factor, and the clamp-loading complex. The core consists
of three subunits: α, the polymerase activity hub, ɛ, exonucleolytic
proofreader, and θ, which may act as a stabilizer for ɛ. The holoenzyme
contains two cores, one for each strand, the lagging and leading.
The beta sliding clamp processivity factor is also present in
duplicate, one for each core, to create a clamp that encloses DNA
allowing for high processivity.
The third assembly is a seven-subunit (τ2γδδ′χψ) clamp loader complex.
Recent research has classified Family C polymerases as a subcategory of
Family X with no eukaryotic equivalents.
Pol IV
In E. coli, DNA polymerase IV (Pol IV) is an error-prone DNA polymerase involved in non-targeted mutagenesis.
Pol IV is a Family Y polymerase expressed by the dinB gene that is
switched on via SOS induction caused by stalled polymerases at the
replication fork. During SOS induction, Pol IV production is increased
tenfold and one of the functions during this time is to interfere with
Pol III holoenzyme processivity. This creates a checkpoint, stops
replication, and allows time to repair DNA lesions via the appropriate
repair pathway. Another function of Pol IV is to perform translesion synthesis
at the stalled replication fork like, for example, bypassing
N2-deoxyguanine adducts at a faster rate than transversing undamaged
DNA. Cells lacking dinB gene have a higher rate of mutagenesis caused
by DNA damaging agents.
Pol V
DNA polymerase V (Pol V) is a Y-family DNA polymerase that is involved in SOS response and translesion synthesis DNA repair mechanisms.
Transcription of Pol V via the umuDC genes is highly regulated to
produce only Pol V when damaged DNA is present in the cell generating an
SOS response. Stalled polymerases causes RecA to bind to the ssDNA,
which causes the LexA protein to autodigest. LexA then loses its ability
to repress the transcription of the umuDC operon. The same RecA-ssDNA
nucleoprotein posttranslationally modifies the UmuD protein into UmuD'
protein. UmuD and UmuD' form a heterodimer that interacts with UmuC,
which in turn activates umuC's polymerase catalytic activity on damaged
DNA.
In E. coli, a polymerase “tool belt” model for switching pol III with
pol IV at a stalled replication fork, where both polymerases bind
simultaneously to the β-clamp, has been proposed.
However, the involvement of more than one TLS polymerase working in
succession to bypass a lesion has not yet been shown in E. coli.
Moreover, Pol IV can catalyze both insertion and extension with high
efficiency, whereas pol V is considered the major SOS TLS polymerase.
One example is the bypass of intra strand guanine thymine cross-link
where it was shown on the basis of the difference in the mutational
signatures of the two polymerases, that pol IV and pol V compete for TLS
of the intra-strand crosslink.
Family D
In 1998, the family D of DNA polymerase was discovered in Pyrococcus furiosus and Methanococcus jannaschii.
The PolD complex is a heterodimer of two chains, each encoded by DP1
(small proofreading) and DP2 (large catalytic). Unlike other DNA
polymerases, the structure and mechanism of the catalytic core resemble
that of multi-subunit RNA polymerases. The DP1-DP2 interface resembles that of Eukaryotic Class B polymerase zinc finger and its small subunit. DP1, a Mre11-like exonuclease, is likely the precursor of small subunit of Pol α and ε, providing proofreading capablities now lost in Eukaryotes. Its N-terminal HSH domain is similar to AAA proteins, especially Pol III subunit δ and RuvB, in structure. DP2 has a Class II KH domain. Pyrococcus abyssi polD is more heat-stable and more accurate than Taq polymerase, but has not yet been commercialized.
Eukaryotic DNA polymerase
Polymerases β, λ, σ and μ (beta, lambda, sigma, and mu)
Family X polymerases contain the well-known eukaryotic polymerase pol β (beta), as well as other eukaryotic polymerases such as Pol σ (sigma), Pol λ (lambda), Pol μ (mu), and Terminal deoxynucleotidyl transferase (TdT).
Family X polymerases are found mainly in vertebrates, and a few are
found in plants and fungi. These polymerases have highly conserved
regions that include two helix-hairpin-helix motifs that are imperative
in the DNA-polymerase interactions. One motif is located in the 8 kDa
domain that interacts with downstream DNA and one motif is located in
the thumb domain that interacts with the primer strand. Pol β, encoded
by POLB gene, is required for short-patch base excision repair, a DNA repair pathway that is essential for repairing alkylated or oxidized bases as well as abasic sites. Pol λ and Pol μ, encoded by the POLL and POLM genes respectively, are involved in non-homologous end-joining,
a mechanism for rejoining DNA double-strand breaks due to hydrogen
peroxide and ionizing radiation, respectively. TdT is expressed only in
lymphoid tissue, and adds "n nucleotides" to double-strand breaks
formed during V(D)J recombination to promote immunological diversity.
Polymerases α, δ and ε (alpha, delta, and epsilon)
Pol α (alpha), Pol δ (delta), and Pol ε (epsilon)
are members of Family B Polymerases and are the main polymerases
involved with nuclear DNA replication. Pol α complex (pol α-DNA primase
complex) consists of four subunits: the catalytic subunit POLA1, the regulatory subunit POLA2, and the small and the large primase subunits PRIM1 and PRIM2 respectively. Once primase has created the RNA primer, Pol α starts replication elongating the primer with ~20 nucleotides. Due to its high processivity, Pol δ takes over the leading and lagging strand synthesis from Pol α. Pol δ is expressed by genes POLD1, creating the catalytic subunit, POLD2, POLD3, and POLD4 creating the other subunits that interact with Proliferating Cell Nuclear Antigen (PCNA), which is a DNA clamp that allows Pol δ to possess processivity. Pol ε is encoded by the POLE1, the catalytic subunit, POLE2, and POLE3 gene. It has been reported that the function of Pol ε is to extend the leading strand during replication,
while Pol δ primarily replicates the lagging strand; however, recent
evidence suggested that Pol δ might have a role in replicating the
leading strand of DNA as well. Pol ε's C-terminus "polymerase relic" region, despite being unnecessary for polymerase activity,
is thought to be essential to cell vitality. The C-terminus region is
thought to provide a checkpoint before entering anaphase, provide
stability to the holoenzyme, and add proteins to the holoenzyme
necessary for initiation of replication. Pol ε has a larger "palm" domain that provides high processivity independtly of PCNA.
Compared to other Family B polymerases, the DEDD exonuclease family responsible for proofreading is inactivated in Pol α.
Pol ε is unique in that it has two zinc finger domains and an inactive
copy of another family B polymerase in its C-terminal. The presence of
this zinc finger has implications in the origins of Eukaryota, which in
this case is placed into the Asgard group with archaeal B3 polymerase.
Polymerases η, ι and κ (eta, iota, and kappa)
Pol η (eta),
Pol ι (iota), and Pol κ (kappa), are Family Y DNA polymerases involved
in the DNA repair by translesion synthesis and encoded by genes POLH, POLI, and POLK
respectively. Members of Family Y have five common motifs to aid in
binding the substrate and primer terminus and they all include the
typical right hand thumb, palm and finger domains with added domains
like little finger (LF), polymerase-associated domain (PAD), or wrist.
The active site, however, differs between family members due to the
different lesions being repaired. Polymerases in Family Y are
low-fidelity polymerases, but have been proven to do more good than harm
as mutations that affect the polymerase can cause various diseases,
such as skin cancer and Xeroderma Pigmentosum Variant (XPS).
The importance of these polymerases is evidenced by the fact that gene
encoding DNA polymerase η is referred as XPV, because loss of this gene
results in the disease Xeroderma Pigmentosum Variant. Pol η is
particularly important for allowing accurate translesion synthesis of
DNA damage resulting from ultraviolet radiation.
The functionality of Pol κ is not completely understood, but
researchers have found two probable functions. Pol κ is thought to act
as an extender or an inserter of a specific base at certain DNA lesions.
All three translesion synthesis polymerases, along with Rev1, are
recruited to damaged lesions via stalled replicative DNA polymerases.
There are two pathways of damage repair leading researchers to conclude
that the chosen pathway depends on which strand contains the damage, the
leading or lagging strand.
Polymerases Rev1 and ζ (zeta)
Pol ζ another B family polymerase, is made of two subunits Rev3, the catalytic subunit, and Rev7 (MAD2L2),
which increases the catalytic function of the polymerase, and is
involved in translesion synthesis. Pol ζ lacks 3' to 5' exonuclease
activity, is unique in that it can extend primers with terminal
mismatches. Rev1
has three regions of interest in the BRCT domain, ubiquitin-binding
domain, and C-terminal domain and has dCMP transferase ability, which
adds deoxycytidine opposite lesions that would stall replicative
polymerases Pol δ and Pol ε. These stalled polymerases activate
ubiquitin complexes that in turn disassociate replication polymerases
and recruit Pol ζ and Rev1. Together Pol ζ and Rev1 add deoxycytidine
and Pol ζ extends past the lesion. Through a yet undetermined process,
Pol ζ disassociates and replication polymerases reassociate and continue
replication. Pol ζ and Rev1 are not required for replication, but loss
of REV3 gene in budding yeast can cause increased sensitivity to
DNA-damaging agents due to collapse of replication forks where
replication polymerases have stalled.
Telomerase
Telomerase is a ribonucleoprotein recruited to replicate ends of linear chromosomes because normal DNA polymerase cannot replicate the ends, or telomere.
The single-strand 3' overhang of the double-strand chromosome with the
sequence 5'-TTAGGG-3' recruits telomerase. Telomerase acts like other
DNA polymerases by extending the 3' end, but, unlike other DNA
polymerases, telomerase does not require a template. The TERT subunit,
an example of a reverse transcriptase,
uses the RNA subunit to form the primer–template junction that allows
telomerase to extend the 3' end of chromosome ends. The gradual decrease
in size of telomeres as the result of many replications over a lifetime
are thought to be associated with the effects of aging.
Polymerases γ, θ and ν (gamma, theta and nu)
Pol γ (gamma), Pol θ (theta), and Pol ν (nu) are Family A polymerases. Pol γ, encoded by the POLG gene, is the only mtDNA
polymerase and therefore replicates, repairs, and has proofreading
3'–5' exonuclease and 5' dRP lyase activities. Any mutation that leads
to limited or non-functioning Pol γ has a significant effect on mtDNA
and is the most common cause of autosomal inherited mitochondrial
disorders.
Pol γ contains a C-terminus polymerase domain and an N-terminus 3'–5'
exonuclease domain that are connected via the linker region, which binds
the accessory subunit. The accessory subunit binds DNA and is required
for processivity of Pol γ. Point mutation A467T in the linker region is
responsible for more than one-third of all Pol γ-associated
mitochondrial disorders. While many homologs of Pol θ, encoded by the POLQ
gene, are found in eukaryotes, its function is not clearly understood.
The sequence of amino acids in the C-terminus is what classifies Pol θ
as Family A polymerase, although the error rate for Pol θ is more
closely related to Family Y polymerases. Pol θ extends mismatched
primer termini and can bypass abasic sites by adding a nucleotide. It
also has Deoxyribophosphodiesterase (dRPase) activity in the polymerase
domain and can show ATPase activity in close proximity to ssDNA. Pol ν (nu) is considered to be the least effective of the polymerase enzymes. However, DNA polymerase nu plays an active role in homology repair during cellular responses to crosslinks, fulfilling its role in a complex with helicase.
Plants use two Family A polymerases to copy both the
mitochrondrial and plastid genomes. They are more similar to bacterial
Pol I than they are to mamallian Pol γ.
Reverse transcriptase
Retroviruses encode an unusual DNA polymerase called reverse transcriptase,
which is an RNA-dependent DNA polymerase (RdDp) that synthesizes DNA
from a template of RNA. The reverse transcriptase family contain both
DNA polymerase functionality and RNase H functionality, which degrades
RNA base-paired to DNA. An example of a retrovirus is HIV.