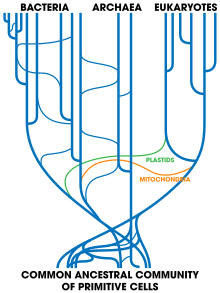
Tree of life showing vertical and horizontal gene transfers
Horizontal gene transfer (HGT) or lateral gene transfer (LGT) is the movement of genetic material between unicellular and/or multicellular organisms other than by the ("vertical") transmission of DNA from parent to offspring (reproduction). HGT is an important factor in the evolution of many organisms.
Horizontal gene transfer is the primary mechanism for the spread of antibiotic resistance in bacteria, and plays an important role in the evolution of bacteria that can degrade novel compounds such as human-created pesticides and in the evolution, maintenance, and transmission of virulence. It often involves temperate bacteriophages and plasmids.
Genes responsible for antibiotic resistance in one species of bacteria
can be transferred to another species of bacteria through various
mechanisms of HGT such as transformation, transduction and conjugation,
subsequently arming the antibiotic resistant genes' recipient against
antibiotics. The rapid spread of antibiotic resistance genes in this
manner is becoming medically challenging to deal with. Ecological
factors may also play a role in the LGT of antibiotic resistant genes.
It is also postulated that HGT promotes the maintenance of a universal
life biochemistry and, subsequently, the universality of the genetic
code.
Most thinking in genetics
has focused upon vertical transfer, but the importance of horizontal
gene transfer among single-cell organisms is beginning to be
acknowledged.
Gene delivery can be seen as an artificial horizontal gene transfer, and is a form of genetic engineering.
History
Griffith's experiment, reported in 1928 by Frederick Griffith, was the first experiment suggesting that bacteria are capable of transferring genetic information through a process known as transformation. Griffith's findings were followed by research in the late 1930s and early 40s that isolated DNA as the material that communicated this genetic information.
Horizontal genetic transfer was then described in Seattle in
1951, in a paper demonstrating that the transfer of a viral gene into Corynebacterium diphtheriae created a virulent strain from a non-virulent strain, also simultaneously solving the riddle of diphtheria (that patients could be infected with the bacteria but not have any symptoms, and then suddenly convert later or never), and giving the first example for the relevance of the lysogenic cycle.
Inter-bacterial gene transfer was first described in Japan in a 1959
publication that demonstrated the transfer of antibiotic resistance
between different species of bacteria. In the mid-1980s, Syvanen
predicted that lateral gene transfer existed, had biological
significance, and was involved in shaping evolutionary history from the
beginning of life on Earth.
As Jian, Rivera and Lake (1999) put it: "Increasingly, studies of
genes and genomes are indicating that considerable horizontal transfer
has occurred between prokaryotes". The phenomenon appears to have had some significance for unicellular eukaryotes
as well. As Bapteste et al. (2005) observe, "additional evidence
suggests that gene transfer might also be an important evolutionary
mechanism in protist evolution."
Grafting of one plant to another can transfer chloroplasts (organelles in plant cells that conduct photosynthesis), mitochondrial DNA, and the entire cell nucleus containing the genome to potentially make a new species. Some Lepidoptera (e.g. monarch butterflies and silkworms) have been genetically modified by horizontal gene transfer from the wasp bracovirus. Bites from the insect Reduviidae (assassin bug) can, via a parasite, infect humans with the trypanosomal Chagas disease, which can insert its DNA into the human genome. It has been suggested that lateral gene transfer to humans from bacteria may play a role in cancer.
Aaron Richardson and Jeffrey D. Palmer
state: "Horizontal gene transfer (HGT) has played a major role in
bacterial evolution and is fairly common in certain unicellular
eukaryotes. However, the prevalence and importance of HGT in the
evolution of multicellular eukaryotes remain unclear."
Due to the increasing amount of evidence suggesting the importance of these phenomena for evolution molecular biologists such as Peter Gogarten have described horizontal gene transfer as "A New Paradigm for Biology".
Mechanisms
There are several mechanisms for horizontal gene transfer:
- Transformation, the genetic alteration of a cell resulting from the introduction, uptake and expression of foreign genetic material (DNA or RNA). This process is relatively common in bacteria, but less so in eukaryotes. Transformation is often used in laboratories to insert novel genes into bacteria for experiments or for industrial or medical applications. See also molecular biology and biotechnology.
- Transduction, the process in which bacterial DNA is moved from one bacterium to another by a virus (a bacteriophage, or phage).
- Bacterial conjugation, a process that involves the transfer of DNA via a plasmid from a donor cell to a recombinant recipient cell during cell-to-cell contact.
- Gene transfer agents, virus-like elements encoded by the host that are found in the alphaproteobacteria order Rhodobacterales.
Horizontal transposon transfer
A transposable element
(TE) (also called a transposon or jumping gene) is a mobile segment of
DNA that can sometimes pick up a resistance gene and insert it into a
plasmid or chromosome, thereby inducing horizontal gene transfer of
antibiotic resistance.
Horizontal transposon transfer (HTT) refers to the passage of
pieces of DNA that are characterized by their ability to move from one locus
to another between genomes by means other than parent-to-offspring
inheritance. Horizontal gene transfer has long been thought to be
crucial to prokaryotic evolution, but there is a growing amount of data
showing that HTT is a common and widespread phenomenon in eukaryote evolution as well.
On the transposable element side, spreading between genomes via
horizontal transfer may be viewed as a strategy to escape purging due to
purifying selection, mutational decay and/or host defense mechanisms.
HTT can occur with any type of transposable elements, but DNA transposons and LTR retroelements
are more likely to be capable of HTT because both have a stable,
double-stranded DNA intermediate that is thought to be sturdier than the
single-stranded RNA intermediate of non-LTR retroelements, which can be highly degradable. Non-autonomous elements may be less likely to transfer horizontally compared to autonomous elements
because they do not encode the proteins required for their own
mobilization. The structure of these non-autonomous elements generally
consists of an intronless gene encoding a transposase
protein, and may or may not have a promoter sequence. Those that do not
have promoter sequences encoded within the mobile region rely on
adjacent host promoters for expression. Horizontal transfer is thought to play an important role in the TE life cycle.
HTT has been shown to occur between species and across continents in both plants
and animals (Ivancevic et al. 2013), though some TEs have been shown to
more successfully colonize the genomes of certain species over others. Both spatial and taxonomic proximity of species has been proposed to favor HTTs in plants and animals.
It is unknown how the density of a population may affect the rate of
HTT events within a population, but close proximity due to parasitism and cross contamination due to crowding have been proposed to favor HTT in both plants and animals.
Successful transfer of a transposable element requires delivery of DNA
from donor to host cell (and to the germ line for multi-cellular
organisms), followed by integration into the recipient host genome. Though the actual mechanism for the transportation of TEs from donor cells to host cells is unknown, it is established that naked DNA and RNA can circulate in bodily fluid. Many proposed vectors include arthropods, viruses, freshwater snails (Ivancevic et al. 2013), endosymbiotic bacteria, and intracellular parasitic bacteria. In some cases, even TEs facilitate transport for other TEs.
The arrival of a new TE in a host genome can have detrimental
consequences because TE mobility may induce mutation. However, HTT can
also be beneficial by introducing new genetic material into a genome and
promoting the shuffling of genes and TE domains among hosts, which can
be co-opted by the host genome to perform new functions. Moreover, transposition activity increases the TE copy number and generates chromosomal rearrangement hotspots.
HTT detection is a difficult task because it is an ongoing phenomenon
that is constantly changing in frequency of occurrence and composition
of TEs inside host genomes. Furthermore, few species have been analyzed
for HTT, making it difficult to establish patterns of HTT events between
species. These issues can lead to the underestimation or overestimation
of HTT events between ancestral and current eukaryotic species.
Methods of detection
Horizontal gene transfer is typically inferred using bioinformatics
methods, either by identifying atypical sequence signatures
("parametric" methods) or by identifying strong discrepancies between
the evolutionary history of particular sequences compared to that of
their hosts. The transferred gene (xenolog) found in the receiving species is more closely related to the genes of the donor species than would be expected.
Viruses
The virus called Mimivirus infects amoebae. Another virus, called Sputnik, also infects amoebae, but it cannot reproduce unless mimivirus has already infected the same cell. "Sputnik's genome
reveals further insight into its biology. Although 13 of its genes show
little similarity to any other known genes, three are closely related
to mimivirus and mamavirus genes, perhaps cannibalized by the tiny virus as it packaged up particles sometime in its history. This suggests that the satellite virus could perform horizontal gene transfer between viruses, paralleling the way that bacteriophages ferry genes between bacteria." Horizontal transfer is also seen between geminiviruses and tobacco plants.
Prokaryotes
Horizontal
gene transfer is common among bacteria, even among very distantly
related ones. This process is thought to be a significant cause of
increased drug resistance when one bacterial cell acquires resistance, and the resistance genes are transferred to other species.
Transposition and horizontal gene transfer, along with strong natural
selective forces have led to multi-drug resistant strains of S. aureus and many other pathogenic bacteria. Horizontal gene transfer also plays a role in the spread of virulence factors, such as exotoxins and exoenzymes, amongst bacteria. A prime example concerning the spread of exotoxins is the adaptive evolution of Shiga toxins in E. coli through horizontal gene transfer via transduction with Shigella species of bacteria.
Strategies to combat certain bacterial infections by targeting these
specific virulence factors and mobile genetic elements have been
proposed. For example, horizontally transferred genetic elements play important roles in the virulence of E. coli, Salmonella, Streptococcus and Clostridium perfringens.
In prokaryotes, restriction-modification systems are known to
provide immunity against horizontal gene transfer and in stabilizing
mobile genetic elements. Genes encoding restriction modification systems
have been reported to move between prokaryotic genomes within mobile genetic elements such as plasmids, prophages, insertion sequences/transposons, integrative conjugative elements (ICEs), and integrons. Still, they are more frequently a chromosomal-encoded barrier to MGEs than an MGE-encoded tool for cell infection.
Bacterial transformation
1:
Donor bacteria 2: Bacteria who will receive the gene 3: The red
portion represents the gene that will be transferred. Transformation
in bacteria in a certain environment.
Natural transformation
is a bacterial adaptation for DNA transfer (HGT) that depends on the
expression of numerous bacterial genes whose products are responsible
for this process.
In general, transformation is a complex, energy-requiring developmental
process. In order for a bacterium to bind, take up and recombine
exogenous DNA into its chromosome, it must become competent, that is, enter a special physiological state. Competence development in Bacillus subtilis requires expression of about 40 genes.
The DNA integrated into the host chromosome is usually (but with
infrequent exceptions) derived from another bacterium of the same species,
and is thus homologous to the resident chromosome. The capacity for
natural transformation occurs in at least 67 prokaryotic species.
Competence for transformation is typically induced by high cell density and/or nutritional limitation, conditions associated with the stationary phase of bacterial growth. Competence appears to be an adaptation for DNA repair.
Transformation in bacteria can be viewed as a primitive sexual process,
since it involves interaction of homologous DNA from two individuals to
form recombinant DNA that is passed on to succeeding generations.
Although transduction is the form of HGT most commonly associated with bacteriophages, certain phages may also be able to promote transformation.
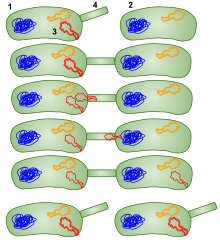
Bacterial conjugation
1:
Donor bacteria cell (F+ cell) 2: Bacteria that receives the plasmid
(F- cell) 3: Plasmid that will be moved to the other bacteria 4:
Pilus. Conjugation in bacteria using a sex pilus; then the bacteria
that received the plasmid can go give it to other bacteria as well.
Conjugation in Mycobacterium smegmatis, like conjugation in E. coli, requires stable and extended contact between a donor and a recipient strain, is DNase resistant, and the transferred DNA is incorporated into the recipient chromosome by homologous recombination. However, unlike E. coli high frequency of recombination conjugation (Hfr), mycobacterial conjugation is a type of HGT that is chromosome rather than plasmid based. Furthermore, in contrast to E. coli (Hfr) conjugation, in M. smegmatis
all regions of the chromosome are transferred with comparable
efficiencies. Substantial blending of the parental genomes was found as a
result of conjugation, and this blending was regarded as reminiscent of
that seen in the meiotic products of sexual reproduction.
Archaeal DNA transfer
The archaeon Sulfolobus solfataricus, when UV irradiated, strongly induces the formation of type IV pili which then facilitates cellular aggregation. Exposure to chemical agents that cause DNA damage also induces cellular aggregation.
Other physical stressors, such as temperature shift or pH, do not
induce aggregation, suggesting that DNA damage is a specific inducer of
cellular aggregation.
UV-induced cellular aggregation mediates intercellular chromosomal HGT marker exchange with high frequency,
and UV-induced cultures display recombination rates that exceed those
of uninduced cultures by as much as three orders of magnitude. S. solfataricus cells aggregate preferentially with other cells of their own species. Frols et al. and Ajon et al.
suggested that UV-inducible DNA transfer is likely an important
mechanism for providing increased repair of damaged DNA via homologous
recombination. This process can be regarded as a simple form of sexual
interaction.
Another thermophilic species, Sulfolobus acidocaldarius, is able to undergo HGT. S. acidocaldarius can exchange and recombine chromosomal markers at temperatures up to 84oC. UV exposure induces pili formation and cellular aggregation.
Cells with the ability to aggregate have greater survival than mutants
lacking pili that are unable to aggregate. The frequency of
recombination is increased by DNA damage induced by UV-irradiation and by DNA damaging chemicals.
The ups operon, containing five genes, is highly induced by UV irradiation. The proteins encoded by the ups operon are employed in UV-induced pili assembly and cellular aggregation leading to intercellular DNA exchange and homologous recombination. Since this system increases the fitness of S. acidocaldarius cells after UV exposure, Wolferen et al. considered that transfer of DNA likely takes place in order to repair UV-induced DNA damages by homologous recombination.
Eukaryotes
"Sequence comparisons suggest recent horizontal transfer of many genes among diverse species including across the boundaries of phylogenetic
'domains'. Thus determining the phylogenetic history of a species can
not be done conclusively by determining evolutionary trees for single
genes."
Organelle to nuclear genome
- Analysis of DNA sequences suggests that horizontal gene transfer has occurred within eukaryotes from the chloroplast and mitochondrial genomes to the nuclear genome. As stated in the endosymbiotic theory, chloroplasts and mitochondria probably originated as bacterial endosymbionts of a progenitor to the eukaryotic cell.
Bacteria to fungi
- Horizontal transfer occurs from bacteria to some fungi, such as the yeast Saccharomyces cerevisiae.
Bacteria to plants
- Agrobacterium, a pathogenic bacterium that causes cells to proliferate as crown galls and proliferating roots is an example of a bacterium that can transfer genes to plants and this plays an important role in plant evolution.
Endosymbiont to insects and nematodes
- The adzuki bean beetle has acquired genetic material from its (non-beneficial) endosymbiont Wolbachia. New examples have recently been reported demonstrating that Wolbachia bacteria represent an important potential source of genetic material in arthropods and filarial nematodes.
Organelle to organelle
- Mitochondrial genes moved to parasites of the Rafflesiaceae plant family from their hosts and from chloroplasts of a still-unidentified plant to the mitochondria of the bean Phaseolus.
Plant to plant
- Striga hermonthica, a parasitic eudicot, has received a gene from sorghum (Sorghum bicolor) to its nuclear genome. The gene's functionality is unknown.
- A gene that allowed ferns to survive in dark forests came from the hornwort, which grows in mats on streambanks or trees. The neochrome gene arrived about 180 million years ago.
Fungi to insects
- Pea aphids (Acyrthosiphon pisum) contain multiple genes from fungi. Plants, fungi, and microorganisms can synthesize carotenoids, but torulene made by pea aphids is the only carotenoid known to be synthesized by an organism in the animal kingdom.
Human to protozoan
- The malaria pathogen Plasmodium vivax acquired genetic material from humans that might help facilitate its long stay in the body.
Bacteria to insects
- HhMAN1 is a gene in the genome of the coffee borer beetle (Hypothenemus hampei) that resembles bacterial genes, and is thought to be transferred from bacteria in the beetle's gut.
Viruses to plants
- Plants are capable of receiving genetic information from viruses by horizontal gene transfer.
Human genome
- One study identified approximately 100 of humans' approximately 20,000 total genes which likely resulted from horizontal gene transfer, but this number has been challenged by several researchers arguing these candidate genes for HGT are more likely the result of gene loss combined with differences in the rate of evolution.
Bacteria to animals
- Bdelloid rotifers currently hold the 'record' for HGT in animals with ~8% of their genes from bacterial origins. Tardigrades were thought to break the record with 17.5% HGT, but that finding was an artifact of bacterial contamination.
- A study found the genomes of 40 animals (including 10 primates, four Caenorhabditis worms, and 12 Drosophila insects) contained genes which the researchers concluded had been transferred from bacteria and fungi by horizontal gene transfer. The researchers estimated that for some nematodes and Drosophilia insects these genes had been acquired relatively recently.
- A bacteriophage-mediated mechanism transfers genes between prokaryotes and eukaryotes. Nuclear localization signals in bacteriophage terminal proteins (TP) prime DNA replication and become covalently linked to the viral genome. The role of virus and bacteriophages in HGT in bacteria, suggests that TP-containing genomes could be a vehicle of inter-kingdom genetic information transference all throughout evolution.
Plants to animals
- The eastern emerald sea slug Elysia chlorotica has been suggested by FISH analysis to contain photosynthesis-supporting genes obtained from an algae (Vaucheria litorea) in their diet. LGT in Sacoglossa is now thought to be an artifact and no trace of LGT was found upon sequencing the genome of Elysia chlorotica.
Plant-fungus
- Gene transfer between plants and fungi has been posited for a number of cases, including rice (Oryza sativa).
Artificial horizontal gene transfer
Before
it is transformed, a bacterium is susceptible to antibiotics. A plasmid
can be inserted when the bacteria is under stress, and be incorporated
into the bacterial DNA creating antibiotic resistance. When the plasmids
are prepared they are inserted into the bacterial cell by either making
pores in the plasma membrane with temperature extremes and chemical
treatments, or making it semi permeable through the process of
electrophoresis, in which electric currents create the holes in the
membrane. After conditions return to normal the holes in the membrane
close and the plasmids are trapped inside the bacteria where they become
part of the genetic material and their genes are expressed by the
bacteria.
Genetic engineering is essentially horizontal gene transfer, albeit with synthetic expression cassettes. The Sleeping Beauty transposon system
(SB) was developed as a synthetic gene transfer agent that was based on
the known abilities of Tc1/mariner transposons to invade genomes of
extremely diverse species. The SB system has been used to introduce genetic sequences into a wide variety of animal genomes.
Importance in evolution
Horizontal gene transfer is a potential confounding factor in inferring phylogenetic trees based on the sequence of one gene.
For example, given two distantly related bacteria that have exchanged a
gene a phylogenetic tree including those species will show them to be
closely related because that gene is the same even though most other
genes are dissimilar. For this reason it is often ideal to use other
information to infer robust phylogenies such as the presence or absence
of genes or, more commonly, to include as wide a range of genes for
phylogenetic analysis as possible.
For example, the most common gene to be used for constructing phylogenetic relationships in prokaryotes is the 16S ribosomal RNA
gene since its sequences tend to be conserved among members with close
phylogenetic distances, but variable enough that differences can be
measured. However, in recent years it has also been argued that 16s rRNA
genes can also be horizontally transferred. Although this may be
infrequent, the validity of 16s rRNA-constructed phylogenetic trees must
be reevaluated.
Biologist Johann Peter Gogarten
suggests "the original metaphor of a tree no longer fits the data from
recent genome research" therefore "biologists should use the metaphor of
a mosaic to describe the different histories combined in individual
genomes and use the metaphor of a net to visualize the rich exchange and
cooperative effects of HGT among microbes". There exist several methods to infer such phylogenetic networks.
Using single genes as phylogenetic markers, it is difficult to trace organismal phylogeny in the presence of horizontal gene transfer. Combining the simple coalescence model of cladogenesis with rare HGT horizontal gene transfer events suggest there was no single most recent common ancestor that contained all of the genes ancestral to those shared among the three domains of life. Each contemporary molecule has its own history and traces back to an individual molecule cenancestor. However, these molecular ancestors were likely to be present in different organisms at different times."
Challenge to the tree of life
Horizontal gene transfer poses a possible challenge to the concept of the last universal common ancestor (LUCA) at the root of the tree of life first formulated by Carl Woese, which led him to propose the Archaea as a third domain of life. Indeed, it was while examining the new three-domain view of life that horizontal gene transfer arose as a complicating issue: Archaeoglobus fulgidus was seen as an anomaly with respect to a phylogenetic tree based upon the encoding for the enzyme HMGCoA reductase—the
organism in question is a definite Archaean, with all the cell lipids
and transcription machinery that are expected of an Archaean, but whose
HMGCoA genes are of bacterial origin. Scientists are broadly agreed on symbiogenesis, that mitochondria in eukaryotes derived from alpha-proteobacterial cells and that chloroplasts came from ingested cyanobacteria,
and other gene transfers may have affected early eukaryotes. (In
contrast, multicellular eukaryotes have mechanisms to prevent horizontal
gene transfer, including separated germ cells.)
If there had been continued and extensive gene transfer, there would be
a complex network with many ancestors, instead of a tree of life with
sharply delineated lineages leading back to a LUCA. However, a LUCA can be identified, so horizontal transfers must have been relatively limited.
Phylogenetic information in HGT
On
the opposite, it has been remarked that the detection of Horizontal
Gene Transfers could bring valuable phylogenetic and dating information.
The potential of HGT to be used for dating phylogenies has recently been confirmed.
The chromosomal organization of Horizontal Gene Transfer
The
acquisition of new genes has the potential to disorganize the other
genetic elements and hinder the function of the bacterial cell, thus
affecting the competitiveness of bacteria. Consequently, bacterial
adaptation lies in a conflict between the advantages of acquiring
beneficial genes, and the need to maintain the organization of the rest
of its genome. Horizontally transferred genes are typically concentrated
in only ~1% of the chromosome (in regions called hotspots). This
concentration increases with genome size and with the rate of transfer.
Hotspots diversify by rapid gene turnover; their chromosomal
distribution depends on local contexts (neighboring core genes), and
content in mobile genetic elements. Hotspots concentrate most changes in
gene repertoires, reduce the trade-off between genome diversification
and organization, and should be treasure troves of strain-specific
adaptive genes. Most mobile genetic elements and antibiotic resistance
genes are in hotspots, but many hotspots lack recognizable mobile
genetic elements and exhibit frequent homologous recombination at
flanking core genes. Overrepresentation of hotspots with fewer mobile
genetic elements in naturally transformable bacteria suggests that
homologous recombination and horizontal gene transfer are tightly linked
in genome evolution.
Genes
There is evidence for historical horizontal transfer of the following genes:
- Lycopene cyclase for carotenoid biosynthesis, between Chlorobi and Cyanobacteria.
- TetO gen conferring resistance to tetracycline, between Campylobacter jejuni.
- Neochrome, gene in some ferns that enhances their ability to survive in dim light. Believed to have been acquired from algae sometime during the Cretaceous.
- transfer of a cysteine synthase from a bacterium into phytophagous mites and Lepidoptera allowing the detoxification of cyanogenic glucosides produced by host plants.
- The LINE1 sequence has transferred from humans to the gonorrhea bacteria.