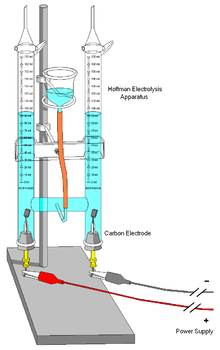
Illustration of an electrolysis apparatus used in a school laboratory
In chemistry and manufacturing, electrolysis is a technique that uses a direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from naturally occurring sources such as ores using an electrolytic cell. The voltage that is needed for electrolysis to occur is called the decomposition potential.
History
The word "electrolysis" was introduced by Michael Faraday in the 19th century, on the suggestion of the Rev. William Whewell, using the Greek words ἤλεκτρον [ɛ̌ːlektron] "amber", which since the 17th century was associated with electric phenomena, and λύσις [lýsis] meaning "dissolution". Nevertheless, electrolysis, as a tool to study chemical reactions and obtain pure elements, precedes the coinage of the term and formal description by Faraday.
- 1785 – Martinus van Marum's electrostatic generator was used to reduce tin, zinc, and antimony from their salts using electrolysis.
- 1800 – William Nicholson and Anthony Carlisle (view also Johann Ritter), decomposed water into hydrogen and oxygen.
- 1808 – Potassium (1807), sodium (1807), barium, calcium and magnesium were discovered by Sir Humphry Davy using electrolysis.
- 1821 – Lithium was discovered by the English chemist William Thomas Brande, who obtained it by electrolysis of lithium oxide.
- 1833 – Michael Faraday develops his two laws of electrolysis, and provides a mathematical explanation of his laws.
- 1875 – Paul Émile Lecoq de Boisbaudran discovered gallium using electrolysis.
- 1886 – Fluorine was discovered by Henri Moissan using electrolysis.
- 1886 – Hall–Héroult process developed for making aluminium
- 1890 – Castner–Kellner process developed for making sodium hydroxide
Overview
Electrolysis is the passing of a direct electric current through an ionic
substance that is either molten or dissolved in a suitable solvent,
producing chemical reactions at the electrodes and a decomposition of
the materials.
The main components required to achieve electrolysis are:
- An electrolyte: a substance, frequently an ion-conducting polymer that contains free ions, which carry electric current in the electrolyte. If the ions are not mobile, as in most solid salts, then electrolysis cannot occur.
- A direct current (DC) electrical supply: provides the energy necessary to create or discharge the ions in the electrolyte. Electric current is carried by electrons in the external circuit.
- Two electrodes: electrical conductors that provide the physical interface between the electrolyte and the electrical circuit that provides the energy.
Electrodes of metal, graphite and semiconductor
material are widely used. Choice of suitable electrode depends on
chemical reactivity between the electrode and electrolyte and
manufacturing cost.
Process of electrolysis
The
key process of electrolysis is the interchange of atoms and ions by the
removal or addition of electrons from the external circuit. The desired
products of electrolysis are often in a different physical state from
the electrolyte and can be removed by some physical processes. For
example, in the electrolysis of brine
to produce hydrogen and chlorine, the products are gaseous. These
gaseous products bubble from the electrolyte and are collected.
- 2 NaCl + 2 H2O → 2 NaOH + H2 + Cl2
A liquid containing electrolyte is produced by:
- Solvation or reaction of an ionic compound with a solvent (such as water) to produce mobile ions
- An ionic compound is melted by heating
An electrical potential is applied across a pair of electrodes immersed in the electrolyte.
Each electrode attracts ions that are of the opposite charge. Positively charged ions (cations) move towards the electron-providing (negative) cathode. Negatively charged ions (anions) move towards the electron-extracting (positive) anode.
In this process electrons
are either absorbed or released. Neutral atoms gain or lose electrons
and become charged ions that then pass into the electrolyte. The
formation of uncharged atoms from ions is called discharging. When an
ion gains or loses enough electrons to become uncharged (neutral) atoms,
the newly formed atoms separate from the electrolyte. Positive metal
ions like Cu2+deposit onto the cathode in a layer. The terms for this are electroplating, electrowinning, and electrorefining.
When an ion gains or loses electrons without becoming neutral, its
electronic charge is altered in the process. In chemistry, the loss of
electrons is called oxidation, while electron gain is called reduction.
Oxidation and reduction at the electrodes
Oxidation of ions or neutral molecules occurs at the anode. For example, it is possible to oxidize ferrous ions to ferric ions at the anode:
- Fe2+(aq) → Fe3+(aq) + e−
It is possible to reduce ferricyanide ions to ferrocyanide ions at the cathode:
- Fe(CN)3-
6 + e− → Fe(CN)4-
6
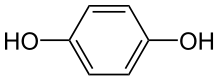
Neutral molecules can also react at either of the electrodes. For
example: p-Benzoquinone can be reduced to hydroquinone at the cathode:
In the last example, H+ ions (hydrogen ions) also take
part in the reaction, and are provided by an acid in the solution, or
by the solvent itself (water, methanol etc.). Electrolysis reactions
involving H+ ions are fairly common in acidic solutions. In aqueous alkaline solutions, reactions involving OH− (hydroxide ions) are common.
Sometimes the solvents themselves (usually water) are oxidized or
reduced at the electrodes. It is even possible to have electrolysis
involving gases. Such as when using a Gas diffusion electrode.
Energy changes during electrolysis
The amount of electrical energy that must be added equals the change in Gibbs free energy of the reaction plus the losses in the system. The losses can (in theory) be arbitrarily close to zero, so the maximum thermodynamic efficiency equals the enthalpy
change divided by the free energy change of the reaction. In most
cases, the electric input is larger than the enthalpy change of the
reaction, so some energy is released in the form of heat. In some cases,
for instance, in the electrolysis of steam
into hydrogen and oxygen at high temperature, the opposite is true and
heat energy is absorbed. This heat is absorbed from the surroundings,
and the heating value of the produced hydrogen is higher than the electric input.
Related techniques
The following techniques are related to electrolysis:
- Electrochemical cells, including the hydrogen fuel cell, use differences in Standard electrode potential to generate an electrical potential that provides useful power. Though related via the interaction of ions and electrolysis and the operation of electrochemical cells are quite distinct. However, a chemical cell should not be seen as performing electrolysis in reverse.
Faraday's laws of electrolysis
First law of electrolysis
In
1832, Michael Faraday reported that the quantity of elements separated
by passing an electric current through a molten or dissolved salt
is proportional to the quantity of electric charge passed through the
circuit. This became the basis of the first law of electrolysis. The
mass of the substance (m) deposited or liberated at any electrode is
directly proportional to the quantity of electricity or charge (Q)
passed. In this equation k is equal to the electromechanical constant.
or
where;
e is known as electrochemical equivalent of the metal deposited or of the gas liberated at the electrode.
Second law of electrolysis
Faraday
discovered that when the same amount of current is passed through
different electrolytes/elements connected in series, the mass of
substance liberated/deposited at the electrodes is directly
proportional to their equivalent weight.
Industrial uses
Hall-Heroult process for producing aluminium
- Electrometallurgy is the process of reduction of metals from metallic compounds to obtain the pure form of metal using electrolysis. Aluminium, lithium, sodium, potassium, magnesium, calcium, and in some cases copper, are produced in this way.
- Production of chlorine and sodium hydroxide
- Production of sodium chlorate and potassium chlorate
- Production of perfluorinated organic compounds such as trifluoroacetic acid by the process of electrofluorination
- Production of electrolytic copper as a cathode, from refined copper of lower purity as an anode.
Electrolysis has many other uses:
- Production of oxygen for spacecraft and nuclear submarines.
- Production of hydrogen for fuel, using a cheap source of electrical energy.
Electrolysis is also used in the cleaning and preservation of old
artifacts. Because the process separates the non-metallic particles from
the metallic ones, it is very useful for cleaning a wide variety of
metallic objects, from old coins to even larger objects including rusted cast iron cylinder blocks and heads when rebuilding automobile engines. Rust removal
from small iron or steel objects by electrolysis can be done in a home
workshop using simple materials such as a plastic bucket, tap water, lengths of rebar, washing soda, baling wire, and a battery charger.
Manufacturing processes
In manufacturing, electrolysis can be used for:
- Electroplating, where a thin film of metal is deposited over a substrate material. Electroplating is used in many industries for either functional or decorative purposes, as in vehicle bodies and nickel coins.
- Electrochemical machining (ECM), where an electrolytic cathode is used as a shaped tool for removing material by anodic oxidation from a workpiece. ECM is often used as technique for deburring or for etching metal surfaces like tools or knives with a permanent mark or logo.
Competing half-reactions in solution electrolysis
Using
a cell containing inert platinum electrodes, electrolysis of aqueous
solutions of some salts leads to reduction of the cations (e.g., metal
deposition with, e.g., zinc salts) and oxidation of the anions (e.g.
evolution of bromine with bromides). However, with salts of some metals
(e.g. sodium) hydrogen is evolved at the cathode, and for salts
containing some anions (e.g. sulfate SO42−) oxygen is evolved at the anode. In both cases this is due to water being reduced to form hydrogen or oxidized to form oxygen.
In principle the voltage required to electrolyze a salt solution can be derived from the standard electrode potential for the reactions at the anode and cathode. The standard electrode potential is directly related to the Gibbs free energy, ΔG, for the reactions at each electrode and refers to an electrode with no current flowing.
In terms of electrolysis, this should be interpreted as follows:
- Oxidized species (often a cation) with a more negative cell potential are more difficult to reduce than oxidized species with a more positive cell potential. For example, it is more difficult to reduce a sodium ion to a sodium metal than it is to reduce a zinc ion to a zinc metal.
- Reduced species (often an anion) with a more positive cell potential are more difficult to oxidize than reduced species with a more negative cell potential. For example, it is more difficult to oxidize sulfate anions than it is to oxidize bromide anions.
Using the Nernst equation the electrode potential can be calculated for a specific concentration of ions, temperature and the number of electrons involved. For pure water (pH 7):
- the electrode potential for the reduction producing hydrogen is −0.41 V
- the electrode potential for the oxidation producing oxygen is +0.82 V.
Comparable figures calculated in a similar way, for 1M zinc bromide, ZnBr2,
are −0.76 V for the reduction to Zn metal and +1.10 V for the oxidation
producing bromine.
The conclusion from these figures is that hydrogen should be produced at
the cathode and oxygen at the anode from the electrolysis of
water—which is at variance with the experimental observation that zinc
metal is deposited and bromine is produced.
The explanation is that these calculated potentials only indicate the
thermodynamically preferred reaction. In practice many other factors
have to be taken into account such as the kinetics of some of the
reaction steps involved. These factors together mean that a higher
potential is required for the reduction and oxidation of water than
predicted, and these are termed overpotentials. Experimentally it is known that overpotentials depend on the design of the cell and the nature of the electrodes.
For the electrolysis of a neutral (pH 7) sodium chloride
solution, the reduction of sodium ion is thermodynamically very
difficult and water is reduced evolving hydrogen leaving hydroxide ions
in solution. At the anode the oxidation of chlorine is observed rather
than the oxidation of water since the overpotential for the oxidation of
chloride to chlorine is lower than the overpotential for the oxidation of water to oxygen. The hydroxide ions and dissolved chlorine gas react further to form hypochlorous acid. The aqueous solutions resulting from this process is called electrolyzed water and is used as a disinfectant and cleaning agent.
Research trends
Electrolysis of carbon dioxide
The electrochemical reduction or electrocatalytic conversion of CO2 can produce value-added chemicals such methane, ethylene, ethane, etc. The electrolysis of carbon dioxide gives formate or carbon monoxide, but sometimes more elaborate organic compounds such as ethylene. This technology is under research as a carbon-neutral route to organic compounds.
Electrolysis of acidified water
Electrolysis of water produces hydrogen.
- 2 H2O(l) → 2 H2(g) + O2(g); E0 = +1.229 V
The energy efficiency
of water electrolysis varies widely. The efficiency of an electrolyser
is a measure of the enthalpy contained in the hydrogen (to undergo
combustion with oxygen, or some other later reaction), compared with the
input electrical energy. Heat/enthalpy values for hydrogen are well
published in science and engineering texts, as 144 MJ/kg. Note that
fuel cells (not electrolysers) cannot use this full amount of
heat/enthalpy, which has led to some confusion when calculating
efficiency values for both types of technology. In the reaction, some
energy is lost as heat. Some reports quote efficiencies between 50% and
70% for alkaline electrolysers; however, much higher practical
efficiencies are available with the use of PEM (Polymer Electrolyte Membrane electrolysis) and catalytic technology, such as 95% efficiency.
NREL
estimated that 1 kg of hydrogen (roughly equivalent to 3 kg, or 4 L, of
petroleum in energy terms) could be produced by wind powered
electrolysis for between $5.55 in the near term and $2.27 in the long
term.
About 4% of hydrogen gas produced worldwide is generated by
electrolysis, and normally used onsite. Hydrogen is used for the
creation of ammonia for fertilizer via the Haber process, and converting heavy petroleum sources to lighter fractions via hydrocracking.
Carbon/hydrocarbon assisted water electrolysis (CAWE)
Recently, to reduce the energy input, the utilization of carbon (coal), alcohols (hydrocarbon solution), and organic solution (glycerol, formic acid, ethylene glycol, etc.) with co-electrolysis of water has been proposed as a viable option.
The carbon/hydrocarbon assisted water electrolysis (so-called CAWE)
process for hydrogen generation would perform this operation in a single
electrochemical
reactor. This system energy balance can be required only around 40%
electric input with 60% coming from the chemical energy of carbon or
hydrocarbon.
This process utilizes solid coal/carbon particles or powder as fuels
dispersed in acid/alkaline electrolyte in the form of slurry and the
carbon contained source co-assist in the electrolysis process as
following theoretical overall reactions:
Carbon/Coal slurry (C + 2H2O) -> CO2 + 2H2 E' = 0.21 V (reversible voltage) / E' = 0.46 V (thermo-neutral voltage)
or
Carbon/Coal slurry (C + H2O) -> CO + H2 E' = 0.52 V reversible voltage) / E' = 0.91 V (thermo-neutral voltage)
Thus, this CAWE approach is that the actual cell overpotential
can be significantly reduced to below 1 V as compared to 1.5 V for
conventional water electrolysis.
Electrocrystallization
A
specialized application of electrolysis involves the growth of
conductive crystals on one of the electrodes from oxidized or reduced
species that are generated in situ. The technique has been used to
obtain single crystals of low-dimensional electrical conductors, such as
charge-transfer salts.
History
Scientific pioneers of electrolysis include:
Pioneers of batteries: