Fire is the rapid oxidation of a material in the exothermic chemical process of combustion, releasing heat, light, and various reaction products. Slower oxidative processes like rusting or digestion are not included by this definition.
Fire is hot because the conversion of the weak double bond in molecular oxygen, O2, to the stronger bonds in the combustion products carbon dioxide and water releases energy (418 kJ per 32 g of O2); the bond energies of the fuel play only a minor role here. At a certain point in the combustion reaction, called the ignition point, flames are produced. The flame
is the visible portion of the fire. Flames consist primarily of carbon
dioxide, water vapor, oxygen and nitrogen. If hot enough, the gases may
become ionized to produce plasma. Depending on the substances alight, and any impurities outside, the color of the flame and the fire's intensity will be different.
Fire in its most common form can result in conflagration, which has the potential to cause physical damage through burning.
Fire is an important process that affects ecological systems around
the globe. The positive effects of fire include stimulating growth and
maintaining various ecological systems.
The negative effects of fire include hazard to life and property, atmospheric pollution, and water contamination. If fire removes protective vegetation, heavy rainfall may lead to an increase in soil erosion by water. Also, when vegetation is burned, the nitrogen it contains is released into the atmosphere, unlike elements such as potassium and phosphorus which remain in the ash
and are quickly recycled into the soil. This loss of nitrogen caused by
a fire produces a long-term reduction in the fertility of the soil,
which only slowly recovers as nitrogen is "fixed" from the atmosphere by lightning and by leguminous plants such as clover.
Fire has been used by humans in rituals, in agriculture for clearing land, for cooking, generating heat and light, for signaling, propulsion purposes, smelting, forging, incineration of waste, cremation, and as a weapon or mode of destruction.
Physical properties
Chemistry
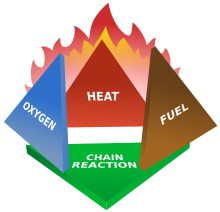
The fire tetrahedron
Fires start when a flammable or a combustible material, in combination with a sufficient quantity of an oxidizer
such as oxygen gas or another oxygen-rich compound (though non-oxygen
oxidizers exist), is exposed to a source of heat or ambient temperature above the flash point for the fuel/oxidizer mix, and is able to sustain a rate of rapid oxidation that produces a chain reaction. This is commonly called the fire tetrahedron.
Fire cannot exist without all of these elements in place and in the
right proportions. For example, a flammable liquid will start burning
only if the fuel and oxygen are in the right proportions. Some
fuel-oxygen mixes may require a catalyst, a substance that is not consumed, when added, in any chemical reaction during combustion, but which enables the reactants to combust more readily.
Once ignited, a chain reaction must take place whereby fires can
sustain their own heat by the further release of heat energy in the
process of combustion and may propagate, provided there is a continuous
supply of an oxidizer and fuel.
If the oxidizer is oxygen from the surrounding air, the presence of a force of gravity, or of some similar force caused by acceleration, is necessary to produce convection,
which removes combustion products and brings a supply of oxygen to the
fire. Without gravity, a fire rapidly surrounds itself with its own
combustion products and non-oxidizing gases from the air, which exclude
oxygen and extinguish the fire. Because of this, the risk of fire in a spacecraft is small when it is coasting in inertial flight. This does not apply if oxygen is supplied to the fire by some process other than thermal convection.
Fire can be extinguished
by removing any one of the elements of the fire tetrahedron. Consider a
natural gas flame, such as from a stove-top burner. The fire can be
extinguished by any of the following:
- turning off the gas supply, which removes the fuel source;
- covering the flame completely, which smothers the flame as the combustion both uses the available oxidizer (the oxygen in the air) and displaces it from the area around the flame with CO2;
- application of water, which removes heat from the fire faster than the fire can produce it (similarly, blowing hard on a flame will displace the heat of the currently burning gas from its fuel source, to the same end), or
- application of a retardant chemical such as Halon to the flame, which retards the chemical reaction itself until the rate of combustion is too slow to maintain the chain reaction.
In contrast, fire is intensified by increasing the overall rate of
combustion. Methods to do this include balancing the input of fuel and
oxidizer to stoichiometric
proportions, increasing fuel and oxidizer input in this balanced mix,
increasing the ambient temperature so the fire's own heat is better able
to sustain combustion, or providing a catalyst, a non-reactant medium
in which the fuel and oxidizer can more readily react.
Flame
Northwest Crown Fire Experiment, Canada
Photo of a fire taken with a 1/4000th of a second exposure
Fire is affected by gravity. Left: Flame on Earth; Right: Flame on the ISS
A flame is a mixture of reacting gases and solids emitting visible, infrared, and sometimes ultraviolet light, the frequency spectrum
of which depends on the chemical composition of the burning material
and intermediate reaction products. In many cases, such as the burning
of organic matter, for example wood, or the incomplete combustion of gas, incandescent solid particles called soot produce the familiar red-orange glow of "fire". This light has a continuous spectrum.
Complete combustion of gas has a dim blue color due to the emission of
single-wavelength radiation from various electron transitions in the
excited molecules formed in the flame. Usually oxygen is involved, but hydrogen burning in chlorine also produces a flame, producing hydrogen chloride (HCl). Other possible combinations producing flames, amongst many, are fluorine and hydrogen, and hydrazine and nitrogen tetroxide. Hydrogen and hydrazine/UDMH flames are similarly pale blue, while burning boron and its compounds, evaluated in mid-20th century as a high energy fuel for jet and rocket engines, emits intense green flame, leading to its informal nickname of "Green Dragon".
The glow of a flame is complex. Black-body radiation
is emitted from soot, gas, and fuel particles, though the soot
particles are too small to behave like perfect blackbodies. There is
also photon emission by de-excited atoms and molecules
in the gases. Much of the radiation is emitted in the visible and
infrared bands. The color depends on temperature for the black-body
radiation, and on chemical makeup for the emission spectra.
The dominant color in a flame changes with temperature. The photo of
the forest fire in Canada is an excellent example of this variation.
Near the ground, where most burning is occurring, the fire is white, the
hottest color possible for organic material in general, or yellow.
Above the yellow region, the color changes to orange, which is cooler,
then red, which is cooler still. Above the red region, combustion no
longer occurs, and the uncombusted carbon particles are visible as black
smoke.
The common distribution of a flame under normal gravity conditions depends on convection, as soot tends to rise to the top of a general flame, as in a candle in normal gravity conditions, making it yellow. In micro gravity or zero gravity, such as an environment in outer space,
convection no longer occurs, and the flame becomes spherical, with a
tendency to become more blue and more efficient (although it may go out
if not moved steadily, as the CO2 from combustion does not
disperse as readily in micro gravity, and tends to smother the flame).
There are several possible explanations for this difference, of which
the most likely is that the temperature is sufficiently evenly
distributed that soot is not formed and complete combustion occurs. Experiments by NASA reveal that diffusion flames
in micro gravity allow more soot to be completely oxidized after they
are produced than diffusion flames on Earth, because of a series of
mechanisms that behave differently in micro gravity when compared to
normal gravity conditions. These discoveries have potential applications in applied science and industry, especially concerning fuel efficiency.
In combustion engines,
various steps are taken to eliminate a flame. The method depends mainly
on whether the fuel is oil, wood, or a high-energy fuel such as jet fuel.
Flame temperatures
Temperatures of flames by appearance
It is true that objects at specific temperatures do radiate visible light.
Objects whose surface is at a temperature above approximately 400 °C
(752 °F) will glow, emitting light at a color that indicates the
temperature of that surface. See the section on red heat
for more about this effect. It is a misconception that one can judge
the temperature of a fire by the color of its flames or the sparks in
the flames. For many reasons, chemically and optically, these colors may
not match the red/orange/yellow/white heat temperatures on the chart. Barium nitrate burns a bright green, for instance, and this is not present on the heat chart.
Typical temperatures of flames
The "adiabatic flame temperature" of a given fuel and oxidizer pair
indicates the temperature at which the gases achieve stable combustion.
- Oxy–dicyanoacetylene 4,990 °C (9,000 °F)
- Oxy–acetylene 3,480 °C (6,300 °F)
- Oxyhydrogen 2,800 °C (5,100 °F)
- Air–acetylene 2,534 °C (4,600 °F)
- Blowtorch (air–MAPP gas) 2,200 °C (4,000 °F)
- Bunsen burner (air–natural gas) 1,300 to 1,600 °C (2,400 to 2,900 °F)
- Candle (air–paraffin) 1,000 °C (1,800 °F)
- Smoldering cigarette:
- Temperature without drawing: side of the lit portion; 400 °C (750 °F); middle of the lit portion: 585 °C (1,100 °F)
- Temperature during drawing: middle of the lit portion: 700 °C (1,300 °F)
- Always hotter in the middle.
Fire ecology
Every natural ecosystem has its own fire regime, and the organisms in those ecosystems are adapted to or dependent upon that fire regime. Fire creates a mosaic of different habitat patches, each at a different stage of succession.
Different species of plants, animals, and microbes specialize in
exploiting a particular stage, and by creating these different types of
patches, fire allows a greater number of species to exist within a
landscape.
Fossil record
The fossil record of fire first appears with the establishment of a land-based flora in the Middle Ordovician period, 470 million years ago, permitting the accumulation of oxygen
in the atmosphere as never before, as the new hordes of land plants
pumped it out as a waste product. When this concentration rose above
13%, it permitted the possibility of wildfire. Wildfire is first recorded in the Late Silurian fossil record, 420 million years ago, by fossils of charcoalified plants. Apart from a controversial gap in the Late Devonian, charcoal is present ever since.
The level of atmospheric oxygen is closely related to the prevalence of
charcoal: clearly oxygen is the key factor in the abundance of
wildfire. Fire also became more abundant when grasses radiated and became the dominant component of many ecosystems, around 6 to 7 million years ago; this kindling provided tinder which allowed for the more rapid spread of fire. These widespread fires may have initiated a positive feedback process, whereby they produced a warmer, drier climate more conducive to fire.
Human control
Bushman starting a fire in Namibia
Process of ignition of a match
The ability to control fire was a dramatic change in the habits of early humans. Making fire to generate heat and light made it possible for people to cook
food, simultaneously increasing the variety and availability of
nutrients and reducing disease by killing organisms in the food. The
heat produced would also help people stay warm in cold weather, enabling
them to live in cooler climates. Fire also kept nocturnal predators at
bay. Evidence of cooked food is found from 1.9 million years ago, although there is a theory that fire could have been used in a controlled fashion about 1 million years ago. Evidence becomes widespread around 50 to 100 thousand years ago, suggesting regular use from this time; resistance to air pollution started to evolve in human populations at a similar point in time.
The use of fire became progressively more sophisticated, with it being
used to create charcoal and to control wildlife from tens of thousands
of years ago.
Fire has also been used for centuries as a method of torture and execution, as evidenced by death by burning as well as torture devices such as the iron boot, which could be filled with water, oil, or even lead and then heated over an open fire to the agony of the wearer.
By the Neolithic Revolution, during the introduction of grain-based agriculture, people all over the world used fire as a tool in landscape management. These fires were typically controlled burns or "cool fires",
as opposed to uncontrolled "hot fires", which damage the soil. Hot
fires destroy plants and animals, and endanger communities. This is
especially a problem in the forests of today where traditional burning
is prevented in order to encourage the growth of timber crops. Cool
fires are generally conducted in the spring and autumn. They clear
undergrowth, burning up biomass
that could trigger a hot fire should it get too dense. They provide a
greater variety of environments, which encourages game and plant
diversity. For humans, they make dense, impassable forests traversable.
Another human use for fire in regards to landscape management is its use
to clear land for agriculture. Slash-and-burn agriculture is still
common across much of tropical Africa, Asia and South America. "For
small farmers, it is a convenient way to clear overgrown areas and
release nutrients from standing vegetation back into the soil", said
Miguel Pinedo-Vasquez, an ecologist at the Earth Institute’s Center for Environmental Research and Conservation.
However this useful strategy is also problematic. Growing population,
fragmentation of forests and warming climate are making the earth's
surface more prone to ever-larger escaped fires. These harm ecosystems
and human infrastructure, cause health problems, and send up spirals of
carbon and soot that may encourage even more warming of the atmosphere –
and thus feed back into more fires. Globally today, as much as 5
million square kilometres – an area more than half the size of the
United States – burns in a given year.
There are numerous modern applications of fire. In its broadest
sense, fire is used by nearly every human being on earth in a controlled
setting every day. Users of internal combustion vehicles employ fire every time they drive. Thermal power stations provide electricity for a large percentage of humanity.
Hamburg after four fire-bombing raids in July 1943, which killed an estimated 50,000 people
The use of fire in warfare has a long history. Fire was the basis of all early thermal weapons. Homer detailed the use of fire by Greek soldiers who hid in a wooden horse to burn Troy during the Trojan war. Later the Byzantine fleet used Greek fire to attack ships and men. In the First World War, the first modern flamethrowers were used by infantry, and were successfully mounted on armoured vehicles in the Second World War. In the latter war, incendiary bombs were used by Axis and Allies alike, notably on Tokyo, Rotterdam, London, Hamburg and, notoriously, at Dresden; in the latter two cases firestorms were deliberately caused in which a ring of fire surrounding each city
was drawn inward by an updraft caused by a central cluster of fires.
The United States Army Air Force also extensively used incendiaries
against Japanese targets in the latter months of the war, devastating
entire cities constructed primarily of wood and paper houses. The use of
napalm was employed in July 1944, towards the end of the Second World War; although its use did not gain public attention until the Vietnam War. Molotov cocktails were also used.
Use as fuel
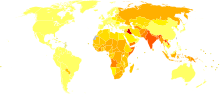
Disability-adjusted life year for fires per 100,000 inhabitants in 2004
no data
less than 50
50–100
100–150
150–200
200–250
250–300
300–350
350–400
400–450
450–500
500–600
more than 600
Setting fuel aflame releases usable energy. Wood was a prehistoric fuel, and is still viable today. The use of fossil fuels, such as petroleum, natural gas, and coal, in power plants supplies the vast majority of the world's electricity today; the International Energy Agency states that nearly 80% of the world's power came from these sources in 2002. The fire in a power station is used to heat water, creating steam that drives turbines. The turbines then spin an electric generator to produce electricity. Fire is also used to provide mechanical work directly, in both external and internal combustion engines.
The unburnable solid remains of a combustible material left after a fire is called clinker if its melting point is below the flame temperature, so that it fuses and then solidifies as it cools, and ash if its melting point is above the flame temperature.
Protection and prevention
Wildfire prevention programs around the world may employ techniques such as wildland fire use and prescribed or controlled burns. Wildland fire use refers to any fire of natural causes that is monitored but allowed to burn. Controlled burns are fires ignited by government agencies under less dangerous weather conditions.
Fire fighting services are provided in most developed areas to extinguish or contain uncontrolled fires. Trained firefighters use fire apparatus, water supply resources such as water mains and fire hydrants or they might use A and B class foam depending on what is feeding the fire.
Fire prevention is intended to reduce sources of ignition. Fire
prevention also includes education to teach people how to avoid causing
fires. Buildings, especially schools and tall buildings, often conduct fire drills to inform and prepare citizens on how to react to a building fire. Purposely starting destructive fires constitutes arson and is a crime in most jurisdictions.
Model building codes require passive fire protection and active fire protection systems to minimize damage resulting from a fire. The most common form of active fire protection is fire sprinklers.
To maximize passive fire protection of buildings, building materials
and furnishings in most developed countries are tested for fire-resistance, combustibility and flammability. Upholstery, carpeting and plastics used in vehicles and vessels are also tested.
Where fire prevention and fire protection have failed to prevent damage, fire insurance can mitigate the financial impact.
Restoration
Different restoration methods and measures are used depending on the
type of fire damage that occurred. Restoration after fire damage can be
performed by property management
teams, building maintenance personnel, or by the homeowners themselves;
however, contacting a certified professional fire damage restoration
specialist is often regarded as the safest way to restore fire damaged
property due to their training and extensive experience.
Most are usually listed under "Fire and Water Restoration" and they can
help speed repairs, whether for individual homeowners or for the
largest of institutions.
Fire and Water Restoration companies are regulated by the
appropriate state's Department of Consumer Affairs – usually the state
contractors license board. In California, all Fire and Water Restoration
companies must register with the California Contractors State License
Board.
Presently, the California Contractors State License Board has no
specific classification for "water and fire damage restoration." Hence,
the Contractor's State License Board requires both an asbestos
certification (ASB) as well as a demolition classification (C-21) in
order to perform Fire and Water Restoration work.