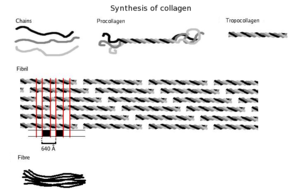
Tropocollagen
molecule: three left-handed procollagens (red, green, blue) join to
form a right-handed triple helical tropocollagen.
Collagen /ˈkɒlədʒɪn/ is the main structural protein in the extracellular matrix in the various connective tissues in the body. As the main component of connective tissue, it is the most abundant protein in mammals, making up from 25% to 35% of the whole-body protein content. Collagen consists of amino acids bound together to form a triple helix of elongated fibril known as a collagen helix. It is mostly found in fibrous tissues such as tendons, ligaments, and skin.
Depending upon the degree of mineralization, collagen tissues may
be rigid (bone), compliant (tendon), or have a gradient from rigid to
compliant (cartilage). It is also abundant in corneas, blood vessels, the gut, intervertebral discs, and the dentin in teeth. In muscle tissue, it serves as a major component of the endomysium. Collagen constitutes one to two percent of muscle tissue and accounts for 6% of the weight of strong, tendinous, muscles. The fibroblast is the most common cell that creates collagen. Gelatin, which is used in food and industry, is collagen that has been irreversibly hydrolyzed. Collagen has many medical uses in treating complications of the bones and skin.
The name collagen comes from the Greek κόλλα (kólla), meaning "glue", and suffix -γέν, -gen, denoting "producing". This refers to the compound's early use in the process of boiling the skin and tendons of horses and other animals to obtain glue.
Types
Over 90% of the collagen in the human body is type I collagen.
However, as of 2011, 28 types of collagen have been identified,
described, and divided into several groups according to the structure
they form: All of the types contain at least one triple helix. The number of types shows collagen's diverse functionality.
- Fibrillar (Type I, II, III, V, XI)
- Non-fibrillar
- FACIT (Fibril Associated Collagens with Interrupted Triple Helices) (Type IX, XII, XIV, XIX, XXI)
- Short chain (Type VIII, X)
- Basement membrane (Type IV)
- Multiplexin (Multiple Triple Helix domains with Interruptions) (Type XV, XVIII)
- MACIT (Membrane Associated Collagens with Interrupted Triple Helices) (Type XIII, XVII)
- Other (Type VI, VII)
The five most common types are:
- Type I: skin, tendon, vasculature, organs, bone (main component of the organic part of bone)
- Type II: cartilage (main collagenous component of cartilage)
- Type III: reticulate (main component of reticular fibers), commonly found alongside type I
- Type IV: forms basal lamina, the epithelium-secreted layer of the basement membrane
- Type V: cell surfaces, hair, and placenta
Medical uses
Cardiac applications
The collagenous cardiac skeleton which includes the four heart valve
rings, is histologically, elastically and uniquely bound to cardiac
muscle. The cardiac skeleton also includes the separating septa of the
heart chambers – the interventricular septum and the atrioventricular septum. Collagen contribution to the measure of cardiac performance summarily represents a continuous torsional force opposed to the fluid mechanics of blood
pressure emitted from the heart. The collagenous structure that divides
the upper chambers of the heart from the lower chambers is an
impermeable membrane that excludes both blood and electrical impulses
through typical physiological means. With support from collagen, atrial fibrillation never deteriorates to ventricular fibrillation.
Collagen is layered in variable densities with cardiac muscle mass. The
mass, distribution, age and density of collagen all contribute to the compliance
required to move blood back and forth. Individual cardiac valvular
leaflets are folded into shape by specialized collagen under variable pressure. Gradual calcium
deposition within collagen occurs as a natural function of aging.
Calcified points within collagen matrices show contrast in a moving
display of blood and muscle, enabling methods of cardiac imaging technology to arrive at ratios essentially stating blood in (cardiac input) and blood out (cardiac output). Pathology of the collagen underpinning of the heart is understood within the category of connective tissue disease.
Cosmetic surgery
Collagen
has been widely used in cosmetic surgery, as a healing aid for burn
patients for reconstruction of bone and a wide variety of dental,
orthopedic, and surgical purposes. Both human and bovine collagen is
widely used as dermal fillers for treatment of wrinkles and skin aging.[11] Some points of interest are:
- When used cosmetically, there is a chance of allergic reactions causing prolonged redness; however, this can be virtually eliminated by simple and inconspicuous patch testing prior to cosmetic use.
- Most medical collagen is derived from young beef cattle (bovine) from certified BSE-free animals. Most manufacturers use donor animals from either "closed herds", or from countries which have never had a reported case of BSE such as Australia, Brazil, and New Zealand.
Bone grafts
As
the skeleton forms the structure of the body, it is vital that it
maintains its strength, even after breaks and injuries. Collagen is used
in bone grafting as it has a triple helical structure, making it a very
strong molecule. It is ideal for use in bones, as it does not
compromise the structural integrity of the skeleton. The triple helical
structure of collagen prevents it from being broken down by enzymes, it
enables adhesiveness of cells and it is important for the proper
assembly of the extracellular matrix.
Tissue regeneration
Collagen
scaffolds are used in tissue regeneration, whether in sponges, thin
sheets, or gels. Collagen has the correct properties for tissue
regeneration such as pore structure, permeability, hydrophilicity, and
being stable in vivo. Collagen scaffolds are also ideal for the
deposition of cells such as osteoblasts and fibroblasts, and once inserted, growth is able to continue as normal in the tissue.
Reconstructive surgical uses
Collagens are widely employed in the construction of the artificial skin substitutes used in the management of severe burns and wounds. These collagens may be derived from bovine, equine, porcine, or even human sources; and are sometimes used in combination with silicones, glycosaminoglycans, fibroblasts, growth factors and other substances.
Wound healing
Collagen is one of the body’s key natural resources and a component of skin tissue that can benefit all stages of wound healing.
When collagen is made available to the wound bed, closure can occur.
Wound deterioration, followed sometimes by procedures such as
amputation, can thus be avoided.
Collagen is a natural product and is thus used as a natural wound
dressing and has properties that artificial wound dressings do not
have. It is resistant against bacteria, which is of vital importance in a
wound dressing. It helps to keep the wound sterile, because of its
natural ability to fight infection. When collagen is used as a burn
dressing, healthy granulation tissue is able to form very quickly over the burn, helping it to heal rapidly.
Throughout the 4 phases of wound healing, collagen performs the following functions in wound healing:
- Guiding function: Collagen fibers serve to guide fibroblasts. Fibroblasts migrate along a connective tissue matrix.
- Chemotactic properties: The large surface area available on collagen fibers can attract fibrogenic cells which help in healing.
- Nucleation: Collagen, in the presence of certain neutral salt molecules can act as a nucleating agent causing formation of fibrillar structures. A collagen wound dressing might serve as a guide for orienting new collagen deposition and capillary growth.
- Hemostatic properties: Blood platelets interact with the collagen to make a hemostatic plug.
As a supplement
When hydrolyzed, collagen is reduced to small peptides, which can be ingested in the form of a dietary supplement or functional foods and beverages with the intent to aid joint and bone health and enhance skin health. Hydrolyzed collagen has a much smaller molecular weight in comparison to native collagen or gelatin.
Studies suggest that more than 90% of hydrolyzed collagen is digested
and available as small peptides in the blood stream within one hour.
From the blood, the peptides (containing hydroxyproline) are transported into the target tissues (e.g., skin, bones, and cartilage), where the peptides act as building blocks for local cells and help boost the production of new collagen fibers.
Basic research
Collagen is used in laboratory studies for cell culture, studying cell behavior and cellular interactions with the extracellular environment.
Veterinary use
Some
studies have shown efficacy of collagen supplementation for dogs with
osteoarthritis pain, alone or in combination with other nutraceuticals
like glucosamine and chondroitin.
Chemistry
The
collagen protein is composed of a triple helix, which generally
consists of two identical chains (α1) and an additional chain that
differs slightly in its chemical composition (α2). The amino acid composition of collagen is atypical for proteins, particularly with respect to its high hydroxyproline content. The most common motifs in the amino acid sequence of collagen are glycine-proline-X
and glycine-X-hydroxyproline, where X is any amino acid other than
glycine, proline or hydroxyproline. The average amino acid composition
for fish and mammal skin is given.
| Amino acid | Abundance in mammal skin (residues/1000) |
Abundance in fish skin (residues/1000) |
|---|---|---|
| Glycine | 329 | 339 |
| Proline | 126 | 108 |
| Alanine | 109 | 114 |
| Hydroxyproline | 95 | 67 |
| Glutamic acid | 74 | 76 |
| Arginine | 49 | 52 |
| Aspartic acid | 47 | 47 |
| Serine | 36 | 46 |
| Lysine | 29 | 26 |
| Leucine | 24 | 23 |
| Valine | 22 | 21 |
| Threonine | 19 | 26 |
| Phenylalanine | 13 | 14 |
| Isoleucine | 11 | 11 |
| Hydroxylysine | 6 | 8 |
| Methionine | 6 | 13 |
| Histidine | 5 | 7 |
| Tyrosine | 3 | 3 |
| Cysteine | 1 | 1 |
| Tryptophan | 0 | 0 |
Synthesis
First,
a three-dimensional stranded structure is assembled, with the amino
acids glycine and proline as its principal components. This is not yet
collagen but its precursor, procollagen. Procollagen is then modified by
the addition of hydroxyl groups to the amino acids proline and lysine. This step is important for later glycosylation
and the formation of the triple helix structure of collagen. Because
the hydroxylase enzymes that perform these reactions require vitamin C as a cofactor, a long-term deficiency in this vitamin results in impaired collagen synthesis and scurvy. These hydroxylation reactions are catalyzed by two different enzymes: prolyl-4-hydroxylase
and lysyl-hydroxylase. Vitamin C also serves with them in inducing
these reactions. In this service, one molecule of vitamin C is destroyed
for each H replaced by OH.
The synthesis of collagen occurs inside and outside of the cell. The
formation of collagen which results in fibrillary collagen (most common
form) is discussed here. Meshwork collagen, which is often involved in
the formation of filtration systems, is the other form of collagen. All
types of collagens are triple helices, and the differences lie in the
make-up of the alpha peptides created in step 2.
- Transcription of mRNA: About 34 genes are associated with collagen formation, each coding for a specific mRNA sequence, and typically have the "COL" prefix. The beginning of collagen synthesis begins with turning on genes which are associated with the formation of a particular alpha peptide (typically alpha 1, 2 or 3).
- Pre-pro-peptide formation: Once the final mRNA exits from the cell nucleus and enters into the cytoplasm, it links with the ribosomal subunits and the process of translation occurs. The early/first part of the new peptide is known as the signal sequence. The signal sequence on the N-terminal of the peptide is recognized by a signal recognition particle on the endoplasmic reticulum, which will be responsible for directing the pre-pro-peptide into the endoplasmic reticulum. Therefore, once the synthesis of new peptide is finished, it goes directly into the endoplasmic reticulum for post-translational processing. It is now known as pre-pro-collagen.
- Pre-pro-peptide to pro-collagen: Three modifications of the pre-pro-peptide occur leading to the formation of the alpha peptide:
- The signal peptide on the N-terminal is dissolved, and the molecule is now known as propeptide (not procollagen).
- Hydroxylation of lysines and prolines on propeptide by the enzymes 'prolyl hydroxylase' and 'lysyl hydroxylase' (to produce hydroxyproline and hydroxylysine) occurs to aid cross-linking of the alpha peptides. This enzymatic step requires vitamin C as a cofactor. In scurvy, the lack of hydroxylation of prolines and lysines causes a looser triple helix (which is formed by three alpha peptides).
- Glycosylation occurs by adding either glucose or galactose monomers onto the hydroxyl groups that were placed onto lysines, but not on prolines.
- Once these modifications have taken place, three of the hydroxylated and glycosylated propeptides twist into a triple helix forming procollagen. Procollagen still has unwound ends, which will be later trimmed. At this point, the procollagen is packaged into a transfer vesicle destined for the Golgi apparatus.
- Golgi apparatus modification: In the Golgi apparatus, the procollagen goes through one last post-translational modification before being secreted out of the cell. In this step, oligosaccharides (not monosaccharides as in step 3) are added, and then the procollagen is packaged into a secretory vesicle destined for the extracellular space.
- Formation of tropocollagen: Once outside the cell, membrane bound enzymes known as collagen peptidases, remove the "loose ends" of the procollagen molecule. What is left is known as tropocollagen. Defects in this step produce one of the many collagenopathies known as Ehlers-Danlos syndrome. This step is absent when synthesizing type III, a type of fibrilar collagen.
- Formation of the collagen fibril: lysyl oxidase, an extracellular copper-dependent enzyme, produces the final step in the collagen synthesis pathway. This enzyme acts on lysines and hydroxylysines producing aldehyde groups, which will eventually undergo covalent bonding between tropocollagen molecules. This polymer of tropocollogen is known as a collagen fibril.
Action of lysyl oxidase
Amino acids
Collagen has an unusual amino acid composition and sequence:
- Glycine is found at almost every third residue.
- Proline makes up about 17% of collagen.
- Collagen contains two uncommon derivative amino acids not directly inserted during translation.
These amino acids are found at specific locations relative to glycine
and are modified post-translationally by different enzymes, both of
which require vitamin C as a cofactor.
- Hydroxyproline derived from proline
- Hydroxylysine derived from lysine - depending on the type of collagen, varying numbers of hydroxylysines are glycosylated (mostly having disaccharides attached).
Cortisol stimulates degradation of (skin) collagen into amino acids.
Collagen I formation
Most collagen forms in a similar manner, but the following process is typical for type I:
- Inside the cell
- Two types of alpha chains are formed during translation on ribosomes along the rough endoplasmic reticulum (RER): alpha-1 and alpha-2 chains. These peptide chains (known as preprocollagen) have registration peptides on each end and a signal peptide.
- Polypeptide chains are released into the lumen of the RER.
- Signal peptides are cleaved inside the RER and the chains are now known as pro-alpha chains.
- Hydroxylation of lysine and proline amino acids occurs inside the lumen. This process is dependent on ascorbic acid (vitamin C) as a cofactor.
- Glycosylation of specific hydroxylysine residues occurs.
- Triple alpha helical structure is formed inside the endoplasmic reticulum from two alpha-1 chains and one alpha-2 chain.
- Procollagen is shipped to the Golgi apparatus, where it is packaged and secreted by exocytosis.
- Outside the cell
- Registration peptides are cleaved and tropocollagen is formed by procollagen peptidase.
- Multiple tropocollagen molecules form collagen fibrils, via covalent cross-linking (aldol reaction) by lysyl oxidase which links hydroxylysine and lysine residues. Multiple collagen fibrils form into collagen fibers.
- Collagen may be attached to cell membranes via several types of protein, including fibronectin, laminin, fibulin and integrin.
Synthetic pathogenesis
Vitamin C deficiency causes scurvy, a serious and painful disease in which defective collagen prevents the formation of strong connective tissue. Gums deteriorate and bleed, with loss of teeth; skin discolors, and wounds
do not heal. Prior to the 18th century, this condition was notorious
among long-duration military, particularly naval, expeditions during
which participants were deprived of foods containing vitamin C.
An autoimmune disease such as lupus erythematosus or rheumatoid arthritis may attack healthy collagen fibers.
Many bacteria and viruses secrete virulence factors, such as the enzyme collagenase, which destroys collagen or interferes with its production.
Molecular structure
A
single collagen molecule, tropocollagen, is used to make up larger
collagen aggregates, such as fibrils. It is approximately 300 nm long and 1.5 nm in diameter, and it is made up of three polypeptide strands (called alpha peptides, see step 2), each of which has the conformation of a left-handed helix – this should not be confused with the right-handed alpha helix. These three left-handed helices are twisted together into a right-handed triple helix or "super helix", a cooperative quaternary structure stabilized by many hydrogen bonds.
With type I collagen and possibly all fibrillar collagens, if not all
collagens, each triple-helix associates into a right-handed
super-super-coil referred to as the collagen microfibril. Each
microfibril is interdigitated
with its neighboring microfibrils to a degree that might suggest they
are individually unstable, although within collagen fibrils, they are so
well ordered as to be crystalline.
Three polypeptides coil to form tropocollagen. Many tropocollagens then bind together to form a fibril, and many of these then form a fibre.
A distinctive feature of collagen is the regular arrangement of amino acids in each of the three chains of these collagen subunits. The sequence often follows the pattern Gly-Pro-X or Gly-X-Hyp, where X may be any of various other amino acid residues.
Proline or hydroxyproline constitute about 1/6 of the total sequence.
With glycine accounting for the 1/3 of the sequence, this means
approximately half of the collagen sequence is not glycine, proline or
hydroxyproline, a fact often missed due to the distraction of the
unusual GX1X2 character of collagen
alpha-peptides. The high glycine content of collagen is important with
respect to stabilization of the collagen helix as this allows the very
close association of the collagen fibers within the molecule,
facilitating hydrogen bonding and the formation of intermolecular
cross-links. This kind of regular repetition and high glycine content is found in only a few other fibrous proteins, such as silk fibroin.
Collagen is not only a structural protein. Due to its key role in
the determination of cell phenotype, cell adhesion, tissue regulation,
and infrastructure, many sections of its non-proline-rich regions have
cell or matrix association/regulation roles. The relatively high content
of proline and hydroxyproline rings, with their geometrically
constrained carboxyl and (secondary) amino
groups, along with the rich abundance of glycine, accounts for the
tendency of the individual polypeptide strands to form left-handed
helices spontaneously, without any intrachain hydrogen bonding.
Because glycine is the smallest amino acid with no side chain, it
plays a unique role in fibrous structural proteins. In collagen, Gly is
required at every third position because the assembly of the triple
helix puts this residue at the interior (axis) of the helix, where there
is no space for a larger side group than glycine’s single hydrogen atom.
For the same reason, the rings of the Pro and Hyp must point outward.
These two amino acids help stabilize the triple helix—Hyp even more so
than Pro; a lower concentration of them is required in animals such as fish, whose body temperatures
are lower than most warm-blooded animals. Lower proline and
hydroxyproline contents are characteristic of cold-water, but not
warm-water fish; the latter tend to have similar proline and
hydroxyproline contents to mammals. The lower proline and hydroxproline contents of cold-water fish and other poikilotherm animals leads to their collagen having a lower thermal stability than mammalian collagen. This lower thermal stability means that gelatin derived from fish collagen is not suitable for many food and industrial applications.
The tropocollagen subunits spontaneously self-assemble, with regularly staggered ends, into even larger arrays in the extracellular spaces of tissues.
Additional assembly of fibrils is guided by fibroblasts, which deposit
fully formed fibrils from fibripositors. In the fibrillar collagens,
molecules are staggered to adjacent molecules by about 67 nm
(a unit that is referred to as ‘D’ and changes depending upon the
hydration state of the aggregate). In each D-period repeat of the
microfibril, there is a part containing five molecules in cross-section,
called the “overlap”, and a part containing only four molecules, called
the "gap".
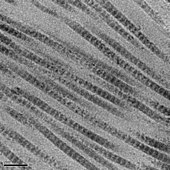
These overlap and gap regions are retained as microfibrils assemble
into fibrils, and are thus viewable using electron microscopy. The
triple helical tropocollagens in the microfibrils are arranged in a
quasihexagonal packing pattern.
The D-period of collagen fibrils results in visible 67nm bands when observed by electron microscopy.
There is some covalent
crosslinking within the triple helices, and a variable amount of
covalent crosslinking between tropocollagen helices forming well
organized aggregates (such as fibrils).
Larger fibrillar bundles are formed with the aid of several different
classes of proteins (including different collagen types), glycoproteins,
and proteoglycans to form the different types of mature tissues from
alternate combinations of the same key players. Collagen's insolubility
was a barrier to the study of monomeric collagen until it was found
that tropocollagen from young animals can be extracted because it is not
yet fully crosslinked.
However, advances in microscopy techniques (i.e. electron microscopy
(EM) and atomic force microscopy (AFM)) and X-ray diffraction have
enabled researchers to obtain increasingly detailed images of collagen
structure in situ. These later advances are particularly
important to better understanding the way in which collagen structure
affects cell–cell and cell–matrix communication and how tissues are
constructed in growth and repair and changed in development and disease.
For example, using AFM–based nanoindentation it has been shown that a
single collagen fibril is a heterogeneous material along its axial
direction with significantly different mechanical properties in its gap
and overlap regions, correlating with its different molecular
organizations in these two regions.
Collagen fibrils/aggregates are arranged in different
combinations and concentrations in various tissues to provide varying
tissue properties. In bone, entire collagen triple helices lie in a
parallel, staggered array. 40 nm gaps between the ends of the
tropocollagen subunits (approximately equal to the gap region) probably
serve as nucleation sites for the deposition of long, hard, fine
crystals of the mineral component, which is hydroxylapatite
(approximately) Ca10(OH)2(PO4)6. Type I collagen gives bone its tensile strength.
Associated disorders
Collagen-related
diseases most commonly arise from genetic defects or nutritional
deficiencies that affect the biosynthesis, assembly, postranslational
modification, secretion, or other processes involved in normal collagen
production.
| Type | Notes | Gene(s) | Disorders |
| I | This is the most abundant collagen of the human body. It is present in scar tissue, the end product when tissue heals by repair. It is found in tendons, skin, artery walls, cornea, the endomysium surrounding muscle fibers, fibrocartilage, and the organic part of bones and teeth. | COL1A1, COL1A2 | Osteogenesis imperfecta, Ehlers–Danlos syndrome, infantile cortical hyperostosis a.k.a. Caffey's disease |
| II | Hyaline cartilage, makes up 50% of all cartilage protein. Vitreous humour of the eye. | COL2A1 | Collagenopathy, types II and XI |
| III | This is the collagen of granulation tissue and is produced quickly by young fibroblasts before the tougher type I collagen is synthesized. Reticular fiber. Also found in artery walls, skin, intestines and the uterus | COL3A1 | Ehlers–Danlos syndrome, Dupuytren's contracture |
| IV | Basal lamina; eye lens. Also serves as part of the filtration system in capillaries and the glomeruli of nephron in the kidney. | COL4A1, COL4A2, COL4A3, COL4A4, COL4A5, COL4A6 | Alport syndrome, Goodpasture's syndrome |
| V | Most interstitial tissue, assoc. with type I, associated with placenta | COL5A1, COL5A2, COL5A3 | Ehlers–Danlos syndrome (classical) |
| VI | Most interstitial tissue, assoc. with type I | COL6A1, COL6A2, COL6A3, COL6A5 | Ulrich myopathy, Bethlem myopathy, atopic dermatitis |
| VII | Forms anchoring fibrils in dermoepidermal junctions | COL7A1 | Epidermolysis bullosa dystrophica |
| VIII | Some endothelial cells | COL8A1, COL8A2 | Posterior polymorphous corneal dystrophy 2 |
| IX | FACIT collagen, cartilage, assoc. with type II and XI fibrils | COL9A1, COL9A2, COL9A3 | EDM2 and EDM3 |
| X | Hypertrophic and mineralizing cartilage | COL10A1 | Schmid metaphyseal dysplasia |
| XI | Cartilage | COL11A1, COL11A2 | Collagenopathy, types II and XI |
| XII | FACIT collagen, interacts with type I containing fibrils, decorin and glycosaminoglycans | COL12A1 | – |
| XIII | Transmembrane collagen, interacts with integrin a1b1, fibronectin and components of basement membranes like nidogen and perlecan. | COL13A1 | – |
| XIV | FACIT collagen, also known as undulin | COL14A1 | – |
| XV | – | COL15A1 | – |
| XVI | – | COL16A1 | – |
| XVII | Transmembrane collagen, also known as BP180, a 180 kDa protein | COL17A1 | Bullous pemphigoid and certain forms of junctional epidermolysis bullosa |
| XVIII | Source of endostatin | COL18A1 | – |
| XIX | FACIT collagen | COL19A1 | – |
| XX | – | COL20A1 | – |
| XXI | FACIT collagen | COL21A1 | – |
| XXII | – | COL22A1 | – |
| XXIII | MACIT collagen | COL23A1 | – |
| XXIV | – | COL24A1 | – |
| XXV | – | COL25A1 | – |
| XXVI | – | EMID2 | – |
| XXVII | – | COL27A1 | – |
| XXVIII | – | COL28A1 | – |
| XXIX | Epidermal collagen | COL29A1 | Atopic dermatitis |
In addition to the above-mentioned disorders, excessive deposition of collagen occurs in scleroderma.
Diseases
One
thousand mutations have been identified in 12 out of more than 20 types
of collagen. These mutations can lead to various diseases at the tissue
level.
Osteogenesis imperfecta – Caused by a mutation in type 1 collagen,
dominant autosomal disorder, results in weak bones and irregular
connective tissue, some cases can be mild while others can be lethal.
Mild cases have lowered levels of collagen type 1 while severe cases
have structural defects in collagen.
Chondrodysplasias – Skeletal disorder believed to be caused by a mutation in type 2 collagen, further research is being conducted to confirm this.
Ehlers-Danlos syndrome – Thirteen different types of this disorder, which lead to deformities in connective tissue, are known.
Some of the rarer types can be lethal, leading to the rupture of
arteries. Each syndrome is caused by a different mutation. For example,
the vascular type (vEDS) of this disorder is caused by a mutation in collagen type 3.
Alport syndrome
– Can be passed on genetically, usually as X-linked dominant, but also
as both an autosomal dominant and autosomal recessive disorder,
sufferers have problems with their kidneys and eyes, loss of hearing can
also develop during the childhood or adolescent years.
Knobloch syndrome – Caused by a mutation in the COL18A1
gene that codes for the production of collagen XVIII. Patients present
with protrusion of the brain tissue and degeneration of the retina; an
individual who has family members suffering from the disorder is at an
increased risk of developing it themselves since there is a hereditary
link.
Characteristics
Collagen is one of the long, fibrous structural proteins whose functions are quite different from those of globular proteins, such as enzymes. Tough bundles of collagen called collagen fibers are a major component of the extracellular matrix
that supports most tissues and gives cells structure from the outside,
but collagen is also found inside certain cells. Collagen has great tensile strength, and is the main component of fascia, cartilage, ligaments, tendons, bone and skin. Along with elastin and soft keratin, it is responsible for skin strength and elasticity, and its degradation leads to wrinkles that accompany aging. It strengthens blood vessels and plays a role in tissue development. It is present in the cornea and lens of the eye in crystalline
form. It may be one of the most abundant proteins in the fossil record,
given that it appears to fossilize frequently, even in bones from the Mesozoic and Paleozoic.
Uses
Collagen has a wide variety of applications, from food to medical. For instance, it is used in cosmetic surgery and burn surgery. It is widely used in the form of collagen casings for sausages, which are also used in the manufacture of musical strings.
If collagen is subject to sufficient denaturation,
e.g. by heating, the three tropocollagen strands separate partially or
completely into globular domains, containing a different secondary
structure to the normal collagen polyproline II (PPII), e.g. random coils. This process describes the formation of gelatin, which is used in many foods, including flavored gelatin desserts. Besides food, gelatin has been used in pharmaceutical, cosmetic, and photography industries.
From the Greek for glue, kolla, the word collagen means "glue producer" and refers to the early process of boiling the skin and sinews of horses and other animals to obtain glue. Collagen adhesive was used by Egyptians about 4,000 years ago, and Native Americans used it in bows about 1,500 years ago. The oldest glue in the world, carbon-dated as more than 8,000 years old, was found to be collagen—used as a protective lining on rope baskets and embroidered fabrics, and to hold utensils together; also in crisscross decorations on human skulls. Collagen normally converts to gelatin, but survived due to dry conditions. Animal glues are thermoplastic, softening again upon reheating, so they are still used in making musical instruments such as fine violins and guitars, which may have to be reopened for repairs—an application incompatible with tough, synthetic plastic adhesives, which are permanent. Animal sinews and skins, including leather, have been used to make useful articles for millennia.
Gelatin-resorcinol-formaldehyde glue (and with formaldehyde replaced by less-toxic pentanedial and ethanedial) has been used to repair experimental incisions in rabbit lungs.
History
The
molecular and packing structures of collagen have eluded scientists over
decades of research. The first evidence that it possesses a regular
structure at the molecular level was presented in the mid-1930s. Since that time, many prominent scholars, including Nobel laureates Crick, Pauling, Rich and Yonath, and others, including Brodsky, Berman, and Ramachandran, concentrated on the conformation of the collagen monomer.
Several competing models, although correctly dealing with the
conformation of each individual peptide chain, gave way to the
triple-helical "Madras" model of Ramachandran, which provided an
essentially correct model of the molecule's quaternary structure although this model still required some refinement. The packing structure of collagen has not been defined to the same degree outside of the fibrillar collagen types, although it has been long known to be hexagonal or quasi-hexagonal.
As with its monomeric structure, several conflicting models alleged
that either the packing arrangement of collagen molecules is
'sheet-like' or microfibrillar.
The microfibrillar structure of collagen fibrils in tendon, cornea and
cartilage has been directly imaged by electron microscopy.
The microfibrillar structure of tail tendon, as described by Fraser,
Miller, and Wess (amongst others), was modeled as being closest to the
observed structure,
although it oversimplified the topological progression of neighboring
collagen molecules, and hence did not predict the correct conformation
of the discontinuous D-periodic pentameric arrangement termed simply:
the microfibril. Various cross linking agents like L-Dopaquinone,
embeline, potassium embelate and 5-O-methyl embelin could be developed
as potential
cross-linking/stabilization agents of collagen preparation and its
application as wound dressing sheet in clinical applications is
enhanced.
The evolution of collagens was a fundamental step in the early evolution of life, supporting the coalescence of multicellular life forms.
D-banding
Collagen D-banding is viable as periodic formation of ridging on all fibrils forming collagen.
D-bands are created due to the semi-crystalline formation of the
collagen within the fibrils. The pattern exhibited by D-banding is
consistently independent of fibril diameter.
When undergoing deformation, collagen fibrils may lose their
D-banding, making the disappearance of the d-bands an indicator of the
type of damage undergone by then tendon fibrils.