Anacortes Refinery (Marathon), on the north end of March Point southeast of Anacortes, Washington, United States
A petrochemical refinery in Grangemouth, Scotland.
An oil refinery or petroleum refinery is an industrial process plant where crude oil is transformed and refined into more useful products such as petroleum naphtha, gasoline, diesel fuel, asphalt base, heating oil, kerosene, liquefied petroleum gas, jet fuel and fuel oils.
Petrochemicals feed stock like ethylene and propylene can also be
produced directly by cracking crude oil without the need of using
refined products of crude oil such as naphtha.
Oil refineries are typically large, sprawling industrial complexes with extensive piping running throughout, carrying streams of fluids between large chemical processing units, such as distillation columns. In many ways, oil refineries use much of the technology, and can be thought of, as types of chemical plants.
The crude oil feedstock has typically been processed by an oil production plant. There is usually an oil depot at or near an oil refinery for the storage of incoming crude oil feedstock as well as bulk liquid products.
Petroleum refineries are very large industrial complexes that
involve many different processing units and auxiliary facilities such as
utility units and storage tanks. Each refinery has its own unique
arrangement and combination of refining processes largely determined by
the refinery location, desired products and economic considerations.
An oil refinery is considered an essential part of the downstream side of the petroleum industry.
Some modern petroleum refineries process as much as 800,000 to
900,000 barrels (127,000 to 143,000 cubic meters) of crude oil per day.
According to the Oil and Gas Journal in the world a total of 636
refineries were operated on the 31 December 2014 for a total capacity of
87.75 million barrels (13,951,000 m3).
Jamnagar Refinery is the largest oil refinery, since 25 December 2008, with a processing capacity of 1.24 million barrels (197,000 m3). Located in Gujarat, India, it is owned by Reliance Industries.
History
The Chinese were among the first civilizations to refine oil. As early as the first century, the Chinese were refining crude oil for use as an energy source. Between 512 and 518, in the late Northern Wei Dynasty, the Chinese geographer, writer and politician Li Daoyuan introduced the process of refining oil into various lubricants in his famous work Commentary on the Water Classic.
Crude oil was often distilled by Arab chemists, with clear descriptions given in Arabic handbooks such as those of Muhammad ibn Zakarīya Rāzi (854–925). The streets of Baghdad were paved with tar, derived from petroleum that became accessible from natural fields in the region. In the 9th century, oil fields were exploited in the area around modern Baku, Azerbaijan. These fields were described by the Arab geographer Abu al-Hasan 'Alī al-Mas'ūdī in the 10th century, and by Marco Polo in the 13th century, who described the output of those wells as hundreds of shiploads. Arab and Persian chemists also distilled crude oil in order to produce flammable products for military purposes. Through Islamic Spain, distillation became available in Western Europe by the 12th century.
In the Northern Song Dynasty
(960–1127), a workshop called the "Fierce Oil Workshop", was
established in the city of Kaifeng to produce refined oil for the Song
military as a weapon. The troops would then fill iron cans with refined
oil and throw them toward the enemy troops, causing a fire – effectively
the world's first "fire bomb".
The workshop was one of the world's earliest oil refining factories
where thousands of people worked to produce Chinese oil powered
weaponry.
Prior to the nineteenth century, petroleum was known and utilized in various fashions in Babylon, Egypt, China, Philippines, Rome and Azerbaijan. However, the modern history of the petroleum industry is said to have begun in 1846 when Abraham Gessner of Nova Scotia, Canada devised a process to produce kerosene from coal. Shortly thereafter, in 1854, Ignacy Łukasiewicz began producing kerosene from hand-dug oil wells near the town of Krosno, Poland.
The world's first systematic petroleum refinery was built in Ploiești, Romania in 1856 using the abundant oil available in Romania.
In North America, the first oil well was drilled in 1858 by James Miller Williams in Oil Springs, Ontario, Canada. In the United States, the petroleum industry began in 1859 when Edwin Drake found oil near Titusville, Pennsylvania.
The industry grew slowly in the 1800s, primarily producing kerosene for
oil lamps. In the early twentieth century, the introduction of the
internal combustion engine and its use in automobiles created a market
for gasoline that was the impetus for fairly rapid growth of the
petroleum industry. The early finds of petroleum like those in Ontario
and Pennsylvania were soon outstripped by large oil "booms" in Oklahoma, Texas and California.
Samuel Kier established America's first oil refinery in Pittsburgh on Seventh avenue near Grant Street, in 1853. Polish pharmacist and inventor Ignacy Łukasiewicz established an oil refinery in Jasło, then part of the Austro-Hungarian Empire (now in Poland) in 1854. The first large refinery opened at Ploiești, Romania, in 1856–1857. After being taken over by Nazi Germany, the Ploiești refineries were bombed in Operation Tidal Wave by the Allies during the Oil Campaign of World War II. Another close contender for the title of hosting the world's oldest oil refinery is Salzbergen in Lower Saxony, Germany. Salzbergen's refinery was opened in 1860.
At one point, the refinery in Ras Tanura, Saudi Arabia owned by Saudi Aramco was claimed to be the largest oil refinery in the world. For most of the 20th century, the largest refinery was the Abadan Refinery in Iran. This refinery suffered extensive damage during the Iran–Iraq War. Since 25 December 2008, the world's largest refinery complex is the Jamnagar Refinery Complex, consisting of two refineries side by side operated by Reliance Industries Limited in Jamnagar, India with a combined production capacity of 1,240,000 barrels per day (197,000 m3/d). PDVSA's Paraguaná Refinery Complex in Paraguaná Peninsula, Venezuela with a capacity of 940,000 bbl/d (149,000 m3/d) and SK Energy's Ulsan in South Korea with 840,000 bbl/d (134,000 m3/d) are the second and third largest, respectively.
Prior to World War II in the early 1940s, most petroleum
refineries in the United States consisted simply of crude oil
distillation units (often referred to as atmospheric crude oil
distillation units). Some refineries also had vacuum distillation units as well as thermal cracking units such as visbreakers (viscosity breakers, units to lower the viscosity
of the oil). All of the many other refining processes discussed below
were developed during the war or within a few years after the war. They
became commercially available within 5 to 10 years after the war ended
and the worldwide petroleum industry experienced very rapid growth. The
driving force for that growth in technology and in the number and size
of refineries worldwide was the growing demand for automotive gasoline
and aircraft fuel.
In the United States, for various complex economic and political
reasons, the construction of new refineries came to a virtual stop in
about the 1980s. However, many of the existing refineries in the United
States have revamped many of their units and/or constructed add-on units
in order to: increase their crude oil processing capacity, increase the
octane rating of their product gasoline, lower the sulfur
content of their diesel fuel and home heating fuels to comply with
environmental regulations and comply with environmental air pollution
and water pollution requirements.
ExxonMobil oil refinery in Baton Rouge, Louisiana (the fourth-largest in the United States)
The size of oil refining market in 2017 was valued over USD 6
trillion in 2017 and is set to witness a consumption of over 100 million
barrels per day (MBPD) by 2024. Oil refining market will witness an
appreciable growth because of rapid industrialization and economic
transformation. Changing demographics, growing population and
improvement in living standards across developing nations are some of
factors positively influencing the industry landscape.
Oil refining in the United States
In the 19th century, refineries in the U.S. processed crude oil primarily to recover the kerosene.
There was no market for the more volatile fraction, including gasoline,
which was considered waste and was often dumped directly into the
nearest river. The invention of the automobile shifted the demand to
gasoline and diesel, which remain the primary refined products today.
Today, national and state legislation require refineries to meet
stringent air and water cleanliness standards. In fact, oil companies in
the U.S. perceive obtaining a permit to build a modern refinery to be
so difficult and costly that no new refineries were built (though many
have been expanded) in the U.S. from 1976 until 2014, when the small
Dakota Prairie Refinery in North Dakota began operation. More than half the refineries that existed in 1981 are now closed due to low utilization rates and accelerating mergers.
As a result of these closures total US refinery capacity fell between
1981 and 1995, though the operating capacity stayed fairly constant in
that time period at around 15,000,000 barrels per day (2,400,000 m3/d).
Increases in facility size and improvements in efficiencies have offset
much of the lost physical capacity of the industry. In 1982 (the
earliest data provided), the United States operated 301 refineries with a
combined capacity of 17.9 million barrels (2,850,000 m3) of
crude oil each calendar day. In 2010, there were 149 operable U.S.
refineries with a combined capacity of 17.6 million barrels (2,800,000 m3) per calendar day. By 2014 the number of refinery had reduced to 140 but the total capacity increased to 18.02 million barrels (2,865,000 m3)
per calendar day. Indeed, in order to reduce operating costs and
depreciation, refining is operated in fewer sites but of bigger
capacity.
In 2009 through 2010, as revenue streams in the oil business
dried up and profitability of oil refineries fell due to lower demand
for product and high reserves of supply preceding the economic recession, oil companies began to close or sell the less profitable refineries.
Operation
Raw or unprocessed crude oil is not generally useful in industrial applications, although "light, sweet" (low viscosity, low sulfur)
crude oil has been used directly as a burner fuel to produce steam for
the propulsion of seagoing vessels. The lighter elements, however, form
explosive vapors in the fuel tanks and are therefore hazardous,
especially in warships.
Instead, the hundreds of different hydrocarbon molecules in crude oil
are separated in a refinery into components that can be used as fuels, lubricants, and feedstocks in petrochemical processes that manufacture such products as plastics, detergents, solvents, elastomers, and fibers such as nylon and polyesters.
Petroleum fossil fuels are burned in internal combustion engines to provide power for ships, automobiles, aircraft engines, lawn mowers, dirt bikes, and other machines. Different boiling points allow the hydrocarbons to be separated by distillation.
Since the lighter liquid products are in great demand for use in
internal combustion engines, a modern refinery will convert heavy
hydrocarbons and lighter gaseous elements into these higher value
products.
The oil refinery in Haifa, Israel is capable of processing about 9 million tons (66 million barrels) of crude oil a year. Its two cooling towers are landmarks of the city's skyline.
Oil can be used in a variety of ways because it contains hydrocarbons of varying molecular masses, forms and lengths such as paraffins, aromatics, naphthenes (or cycloalkanes), alkenes, dienes, and alkynes.
While the molecules in crude oil include different atoms such as sulfur
and nitrogen, the hydrocarbons are the most common form of molecules,
which are molecules of varying lengths and complexity made of hydrogen and carbon atoms, and a small number of oxygen atoms. The differences in the structure of these molecules account for their varying physical and chemical properties, and it is this variety that makes crude oil useful in a broad range of several applications.
Once separated and purified of any contaminants and impurities,
the fuel or lubricant can be sold without further processing. Smaller
molecules such as isobutane and propylene or butylenes can be recombined to meet specific octane requirements by processes such as alkylation, or more commonly, dimerization. The octane grade of gasoline can also be improved by catalytic reforming, which involves removing hydrogen from hydrocarbons producing compounds with higher octane ratings such as aromatics. Intermediate products such as gasoils can even be reprocessed to break a heavy, long-chained oil into a lighter short-chained one, by various forms of cracking such as fluid catalytic cracking, thermal cracking, and hydrocracking. The final step in gasoline production is the blending of fuels with different octane ratings, vapor pressures,
and other properties to meet product specifications. Another method for
reprocessing and upgrading these intermediate products (residual oils)
uses a devolatilization process to separate usable oil from the waste asphaltene material.
Oil refineries are large scale plants, processing about a hundred thousand to several hundred thousand barrels of crude oil a day. Because of the high capacity, many of the units operate continuously, as opposed to processing in batches, at steady state or nearly steady state for months to years. The high capacity also makes process optimization and advanced process control very desirable.
Major products
Crude oil is separated into fractions by fractional distillation. The fractions at the top of the fractionating column have lower boiling points than the fractions at the bottom. The heavy bottom fractions are often cracked into lighter, more useful products. All of the fractions are processed further in other refining units.
A breakdown of the products made from a typical barrel of US oil.
Petroleum products are materials derived from crude oil (petroleum) as it is processed in oil refineries. The majority of petroleum is converted to petroleum products, which includes several classes of fuels.
Oil refineries also produce various intermediate products such as hydrogen, light hydrocarbons, reformate and pyrolysis gasoline.
These are not usually transported but instead are blended or processed
further on-site. Chemical plants are thus often adjacent to oil
refineries or a number of further chemical processes are integrated into
it. For example, light hydrocarbons are steam-cracked in an ethylene plant, and the produced ethylene is polymerized to produce polyethene.
Because technical reasons and environment protection demand a
very low sulfur content in all but the heaviest products, it is
transformed to hydrogen sulfide via catalytic hydrodesulfurization and removed from the product stream via amine gas treating. Using the Claus process,
hydrogen sulfide is afterwards transformed to elementary sulfur to be
sold to the chemical industry. The rather large heat energy freed by
this process is directly used in the other parts of the refinery. Often
an electrical power plant is combined into the whole refinery process to
take up the excess heat.
According to the composition of the crude oil and depending on
the demands of the market, refineries can produce different shares of
petroleum products. The largest share of oil products is used as "energy
carriers", i.e. various grades of fuel oil and gasoline. These fuels include or can be blended to give gasoline, jet fuel, diesel fuel, heating oil, and heavier fuel oils. Heavier (less volatile) fractions can also be used to produce asphalt, tar, paraffin wax, lubricating and other heavy oils. Refineries also produce other chemicals, some of which are used in chemical processes to produce plastics and other useful materials. Since petroleum often contains a few percent sulfur-containing molecules, elemental sulfur is also often produced as a petroleum product. Carbon, in the form of petroleum coke, and hydrogen
may also be produced as petroleum products. The hydrogen produced is
often used as an intermediate product for other oil refinery processes
such as hydrocracking and hydrodesulfurization.
Petroleum products are usually grouped into four categories:
light distillates (LPG, gasoline, naphtha), middle distillates
(kerosene, jet fuel, diesel), heavy distillates and residuum (heavy fuel
oil, lubricating oils, wax, asphalt). These require blending various
feedstocks, mixing appropriate additives, providing short term storage,
and preparation for bulk loading to trucks, barges, product ships, and
railcars. This classification is based on the way crude oil is distilled
and separated into fractions.
- Gaseous fuel such as Liquified petroleum gas and propane, stored and shipped in liquid form under pressure.
- Lubricants (produces light machine oils, motor oils, and greases, adding viscosity stabilizers as required), usually shipped in bulk to an offsite packaging plant.
- Paraffin wax, used in the packaging of frozen foods, among others. May be shipped in bulk to a site to prepare as packaged blocks. Used for wax emulsions, construction board, matches, candles, rust protection, and vapor barriers.
- Sulfur (or sulfuric acid), byproducts of sulfur removal from petroleum which may have up to a couple percent sulfur as organic sulfur-containing compounds. Sulfur and sulfuric acid are useful industrial materials. Sulfuric acid is usually prepared and shipped as the acid precursor oleum.
- Bulk tar shipping for offsite unit packaging for use in tar-and-gravel roofing.
- Asphalt used as a binder for gravel to form asphalt concrete, which is used for paving roads, lots, etc. An asphalt unit prepares bulk asphalt for shipment.
- Petroleum coke, used in specialty carbon products like electrodes or as solid fuel.
- Petrochemicals are organic compounds that are the ingredients for the chemical industry, ranging from polymers and pharmaceuticals, including ethylene and benzene-toluene-xylenes ("BTX") which are often sent to petrochemical plants for further processing in a variety of ways. The petrochemicals may be olefins or their precursors, or various types of aromatic petrochemicals.
- Gasoline
- Naphtha
- Kerosene and related jet aircraft fuels
- Diesel fuel and Fuel oils
- Heat
- Electricity
Over 6,000 items are made from petroleum waste by-products including: fertilizer, floor coverings, perfume, insecticide, petroleum jelly, soap, vitamin capsules. See link to partial list of 144 by-products listed by Ranken Energy
- Sample of Crude oil (petroleum)
- Sample of Gasoline
- Sample of Kerosene
- Sample of Diesel fuel
- Pile of asphalt-covered aggregate for formation into asphalt concrete
Chemical processes found in a refinery
Storage tanks and towers at Shell Puget Sound Refinery (Shell Oil Company), Anacortes, Washington
- Desalter unit washes out salt from the crude oil before it enters the atmospheric distillation unit.
- Crude Oil Distillation unit (Atmospheric distillation): Distills the incoming crude oil into various fractions for further processing in other units. See continuous distillation.
- Vacuum distillation further distills the residue oil from the bottom of the crude oil distillation unit. The vacuum distillation is performed at a pressure well below atmospheric pressure.
- Naphtha hydrotreater unit uses hydrogen to desulfurize naphtha from atmospheric distillation. Must hydrotreat the naphtha before sending to a catalytic reformer unit.
- Catalytic reformer converts the desulfurized naphtha molecules into higher-octane molecules to produce reformate (reformer product). The reformate has higher content of aromatics and cyclic hydrocarbons which is a component of the end-product gasoline or petrol. An important byproduct of a reformer is hydrogen released during the catalyst reaction. The hydrogen is used either in the hydrotreaters or the hydrocracker.
- Distillate hydrotreater desulfurizes distillates (such as diesel) after atmospheric distillation. Uses hydrogen to desulfurize the naphtha fraction from the crude oil distillation or other units within the refinery.
- Fluid Catalytic Cracker (FCC) upgrades the heavier, higher-boiling fractions from the crude oil distillation by converting them into lighter and lower boiling, more valuable products.
- Hydrocracker uses hydrogen to upgrade heavy residual oils from the vacuum distillation unit by thermally cracking them into lighter, more valuable reduced viscosity products.
- Merox desulfurize LPG, kerosene or jet fuel by oxidizing mercaptans to organic disulfides.
- Alternative processes for removing mercaptans are known, e.g. doctor sweetening process and caustic washing.
- Coking units (delayed coking, fluid coker, and flexicoker) process very heavy residual oils into gasoline and diesel fuel, leaving petroleum coke as a residual product.
- Alkylation unit uses sulfuric acid or hydrofluoric acid to produce high-octane components for gasoline blending. Converts isobutane and butylenes into alkylate, which is a very high-octane component of the end-product gasoline or petrol.
- Dimerization unit converts olefins into higher-octane gasoline blending components. For example, butenes can be dimerized into isooctene which may subsequently be hydrogenated to form isooctane. There are also other uses for dimerization. Gasoline produced through dimerization is highly unsaturated and very reactive. It tends spontaneously to form gums. For this reason the effluent from the dimerization need to be blended into the finished gasoline pool immediately or hydrogenated.
- Isomerization converts linear molecules such as normal pentane to higher-octane branched molecules for blending into gasoline or feed to alkylation units. Also used to convert linear normal butane into isobutane for use in the alkylation unit.
- Steam reforming converts natural gas into hydrogen for the hydrotreaters and/or the hydrocracker.
- Liquified gas storage vessels store propane and similar gaseous fuels at pressure sufficient to maintain them in liquid form. These are usually spherical vessels or "bullets" (i.e., horizontal vessels with rounded ends).
- Amine gas treater, Claus unit, and tail gas treatment convert hydrogen sulfide from hydrodesulfurization into elemental sulfur. The large majority of the 64,000,000 metric tons of sulfur produced worldwide in 2005 was byproduct sulfur from petroleum refining and natural gas processing plants.
- Sour water stripper Uses steam to remove hydrogen sulfide gas from various wastewater streams for subsequent conversion into end-product sulfur in the Claus unit.
- Cooling towers circulate cooling water, boiler plants generates steam for steam generators, and instrument air systems include pneumatically operated control valves and an electrical substation.
- Wastewater collection and treating systems consist of API separators, dissolved air flotation (DAF) units and further treatment units such as an activated sludge biotreater to make water suitable for reuse or for disposal.
- Solvent refining use solvent such as cresol or furfural to remove unwanted, mainly aromatics from lubricating oil stock or diesel stock.
- Solvent dewaxing remove the heavy waxy constituents petrolatum from vacuum distillation products.
- Liquified gas (LPG) storage vessels for propane and similar gaseous fuels at a pressure sufficient to maintain them in liquid form. These are usually spherical vessels or bullets (horizontal vessels with rounded ends).
- Storage tanks for storing crude oil and finished products, usually vertical, cylindrical vessels with some sort of vapour emission control and surrounded by an earthen berm to contain spills.
Flow diagram of typical refinery
The image below is a schematic flow diagram of a typical oil refinery that depicts the various unit
processes and the flow of intermediate product streams that occurs
between the inlet crude oil feedstock and the final end products. The diagram
depicts only one of the literally hundreds of different oil refinery
configurations. The diagram also does not include any of the usual
refinery facilities providing utilities such as steam, cooling water,
and electric power as well as storage tanks for crude oil feedstock and
for intermediate products and end products.
There are many process configurations other than that depicted above. For example, the vacuum distillation
unit may also produce fractions that can be refined into end products
such as: spindle oil used in the textile industry, light machinery oil,
motor oil, and various waxes.
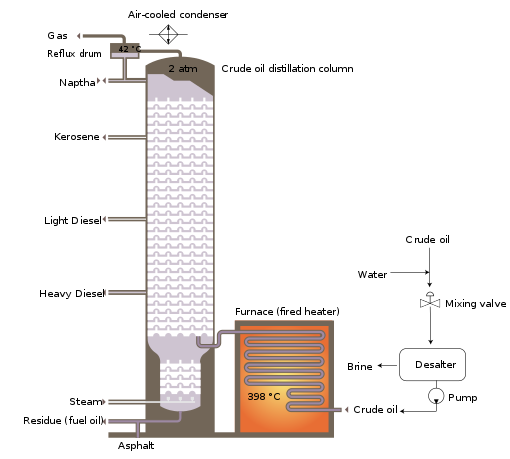
The crude oil distillation unit
The
crude oil distillation unit (CDU) is the first processing unit in
virtually all petroleum refineries. The CDU distills the incoming crude
oil into various fractions of different boiling ranges, each of which
are then processed further in the other refinery processing units. The
CDU is often referred to as the atmospheric distillation unit because it operates at slightly above atmospheric pressure.
Below is a schematic flow diagram of a typical crude oil
distillation unit. The incoming crude oil is preheated by exchanging
heat with some of the hot, distilled fractions and other streams. It is
then desalted to remove inorganic salts (primarily sodium chloride).
Following the desalter, the crude oil is further heated by
exchanging heat with some of the hot, distilled fractions and other
streams. It is then heated in a fuel-fired furnace (fired heater) to a
temperature of about 398 °C and routed into the bottom of the
distillation unit.
The cooling and condensing of the distillation tower overhead is
provided partially by exchanging heat with the incoming crude oil and
partially by either an air-cooled or water-cooled condenser. Additional
heat is removed from the distillation column by a pumparound system as
shown in the diagram below.
As shown in the flow diagram, the overhead distillate fraction
from the distillation column is naphtha. The fractions removed from the
side of the distillation column at various points between the column top
and bottom are called sidecuts. Each of the sidecuts (i.e., the
kerosene, light gas oil and heavy gas oil) is cooled by exchanging heat
with the incoming crude oil. All of the fractions (i.e., the overhead
naphtha, the sidecuts and the bottom residue) are sent to intermediate
storage tanks before being processed further.
Schematic flow diagram of a typical crude oil distillation unit as used in petroleum crude oil refineries.
Location of petroleum refineries
A party searching for a site to construct a refinery or a chemical plant needs to consider the following issues:
- The site has to be reasonably far from residential areas.
- Infrastructure should be available for supply of raw materials and shipment of products to markets.
- Energy to operate the plant should be available.
- Facilities should be available for waste disposal.
Refineries which use a large amount of steam and cooling water need
to have an abundant source of water. Oil refineries therefore are often
located nearby navigable rivers or on a sea shore, nearby a port. Such
location also gives access to transportation by river or by sea. The
advantages of transporting crude oil by pipeline are evident, and oil
companies often transport a large volume of fuel to distribution
terminals by pipeline. Pipeline may not be practical for products with
small output, and rail cars, road tankers, and barges are used.
Petrochemical plants and solvent manufacturing (fine
fractionating) plants need spaces for further processing of a large
volume of refinery products for further processing, or to mix chemical
additives with a product at source rather than at blending terminals.
Safety and environment
Fire-extinguishing operations after the Texas City Refinery explosion.
The refining process releases a number of different chemicals into the atmosphere (see AP 42 Compilation of Air Pollutant Emission Factors) and a notable odor normally accompanies the presence of a refinery. Aside from air pollution impacts there are also wastewater concerns, risks of industrial accidents such as fire and explosion, and noise health effects due to industrial noise.
Many governments worldwide have mandated restrictions on
contaminants that refineries release, and most refineries have installed
the equipment needed to comply with the requirements of the pertinent
environmental protection regulatory agencies. In the United States,
there is strong pressure to prevent the development of new refineries,
and no major refinery has been built in the country since Marathon's Garyville, Louisiana
facility in 1976. However, many existing refineries have been expanded
during that time. Environmental restrictions and pressure to prevent
construction of new refineries may have also contributed to rising fuel
prices in the United States.
Additionally, many refineries (more than 100 since the 1980s) have
closed due to obsolescence and/or merger activity within the industry
itself.
Environmental and safety concerns mean that oil refineries are
sometimes located some distance away from major urban areas.
Nevertheless, there are many instances where refinery operations are
close to populated areas and pose health risks. In California's Contra Costa County and Solano County,
a shoreline necklace of refineries, built in the early 20th century
before this area was populated, and associated chemical plants are adjacent to urban areas in Richmond, Martinez, Pacheco, Concord, Pittsburg, Vallejo and Benicia, with occasional accidental events that require "shelter in place" orders to the adjacent populations. A number of refineries are located in Sherwood Park, Alberta, directly adjacent to the City of Edmonton. The Edmonton metro area has a population of over 1,000,000 residents.
NIOSH criteria for occupational exposure to refined petroleum solvents have been available since 1977.
Worker health
Background
Modern petroleum refining involves a complicated system of interrelated chemical reactions that produce a wide variety of petroleum-based products. Many of these reactions require precise temperature and pressure parameters.
The equipment and monitoring required to ensure the proper progression
of these processes is complex, and has evolved through the advancement
of the scientific field of petroleum engineering.
The wide array of high pressure and/or high temperature
reactions, along with the necessary chemical additives or extracted
contaminants, produces an astonishing number of potential health hazards
to the oil refinery worker.
Through the advancement of technical chemical and petroleum
engineering, the vast majority of these processes are automated and
enclosed, thus greatly reducing the potential health impact to workers.
However, depending on the specific process in which a worker is
engaged, as well as the particular method employed by the refinery in
which he/she works, significant health hazards remain.
Although U.S. occupational injuries were not routinely
tracked/reported at the time, reports of the health impacts of working
in an oil refinery can be found as early as the 1800s. For instance, an
explosion in a Chicago refinery killed 20 workers in 1890.
Since then, numerous fires, explosions, and other significant events
have from time to time drawn the public's attention to the health of oil
refinery workers. Such events continue today, with explosions reported in refineries in Wisconsin and Germany in 2018.
However, there are many less visible hazards that endanger oil refinery workers.
Chemical exposures
Given
the highly automated and technically advanced nature of modern
petroleum refineries, nearly all processes are contained within
engineering controls and represent a substantially decreased risk of
exposure to workers compared to earlier times.
However, certain situations or work tasks may subvert these safety
mechanisms, and expose workers to a number of chemical (see table above)
or physical (described below) hazards. Examples of these scenarios include:
- System failures (leaks, explosions, etc.).
- Standard inspection, product sampling, process turnaround, or equipment maintenance/cleaning activities.
Interestingly, even though petroleum refineries utilize and produce chemicals that are known carcinogens, the literature on cancer rates among refinery workers is mixed. For example, benzene has been shown to have a relationship with leukemia,
however studies examining benzene exposure and resultant leukemia
specifically in the context of oil refinery workers have come to
opposing conclusions. Asbestos-related mesothelioma
is another particular cancer-carcinogen relationship that has been
investigated in the context of oil refinery workers. To date, this work
has shown a marginally significant link to refinery employment and
mesothelioma.
Notably, a meta-analysis which included data on more than 350,000
refinery workers failed to find any statistically significant excess
rates of cancer mortality, except for a marginally significant increase
in melanoma deaths.
An additional U.S.-based study included a follow-up period of 50 years
among over 17,000 workers. This study concluded that there was no
excess mortality among this cohort as a result of employment.
BTX stands for benzene, toluene, xylene. This is a group of common volatile organic compounds
(VOC's) that are found in the oil refinery environment, and serve as a
paradigm for more in depth discussion of occupational exposure limits,
chemical exposure and surveillance among refinery workers.
The most important route of exposure for BTEX chemicals is
inhalation due to the low boiling point of these chemicals. The
majority of the gaseous production of BTEX occurs during tank cleaning
and fuel transfer, which causes offgassing of these chemicals into the
air. Exposure can also occur through ingestion via contaminated water, but this is unlikely in an occupational setting.
Dermal exposure and absorption is also possible, but is again less
likely in an occupational setting where appropriate personal protective
equipment is in place.
OSHA, NIOSH, and ACGIH have all established occupational exposure limits (OEL's) for many of the chemicals above that workers may be exposed to in petroleum refineries.
|
|
OSHA PEL (8-hour TWA) | Cal/OSHA PEL (8-hour TWA) | NIOSH REL (10-hour TWA) | ACGIH TLV (8-hour TWA) |
|---|---|---|---|---|
| Benzene | 10 ppm | 1 ppm | 1 ppm | 0.5 ppm |
| Toluene | 10 ppm | 1 ppm | 10 ppm | 1 ppm |
| Xylene | 100 ppm | 100 ppm | 100 ppm | 100 ppm |
Benzene, in particular, has multiple biomarkers
that can be measured to determine exposure. Benzene itself can be
measured in the breath, blood, and urine, and metabolites such as phenol, t,t-muconic acid (t,tMA) and S-phenylmercapturic acid (sPMA) can be measured in urine.
In addition to monitoring the exposure levels via these biomarkers,
employers are required by OSHA to perform regular blood tests on workers
to test for early signs of some of the feared hematologic outcomes, of
which the most widely recognized is leukemia. Required testing includes complete blood count with cell differentials and peripheral blood smear "on a regular basis". The utility of these tests is supported by formal scientific studies.
Physical hazards
Workers
are at risk of physical injuries due to the large number of
high-powered machines in the relatively close proximity of the oil
refinery. The high pressure required for many of the chemical reactions
also presents the possibility of localized system failures resulting in
blunt or penetrating trauma from exploding system components.
However, Bureau of Labor (BLS) statistical reports indicate that
petroleum refinery workers have a significantly lower rate of
occupational injury (0.7 OSHA-recordable cases per 100 full-time
workers) than all industries (3.1), oil and gas extraction (1.0), and
petroleum manufacturing in general (1.6).
Heat is also a hazard. The temperature required for the proper
progression of certain reactions in the refining process can reach 1600
degrees F.
As with chemicals, the operating system is designed to safely contain
this hazard without injury to the worker. However, in system failures
this is a potent threat to workers’ health. Concerns include both
direct injury through a heat illness or injury, as well as the potential for devastating burns should the worker come in contact with super-heated reagents/equipment.
Noise is another hazard. Refineries can be very loud
environments, and have previously been shown to be associated with
hearing loss among workers. The interior environment of an oil refinery can reach levels in excess of 90 dB. An average of 90 dB is the OSHA Permissible Exposure Limit (PEL) for an 8 hour work-day. Noise exposures that average greater than 85 dB over an 8 hour require a hearing conservation program to regularly evaluate workers' hearing and to promote its protection. Regular evaluation of workers’ auditory capacity and faithful use of properly vetted hearing protection are essential parts of such programs.
While not specific to the industry, oil refinery workers may also be at risk for hazards such as vehicle-related accidents, machinery-associated injuries, work in a confined space, explosions/fires, ergonomic hazards, shift-work related sleep disorders, and falls.
Hazard controls
The theory of hierarchy of controls can be applied to petroleum refineries and their efforts to ensure worker safety.
Elimination and substitution
are unlikely in petroleum refineries, as many of the raw materials,
waste products, and finished products are hazardous in one form or
another (e.g. flammable, carcinogenic).
Examples of engineering controls include a fire detection/extinguishing system, pressure/chemical sensors to detect/predict loss of structural integrity, and adequate maintenance of piping to prevent hydrocarbon-induced corrosion (leading to structural failure). Other examples employed in petroleum refineries include the post-construction protection of steel components with vermiculite to improve heat/fire resistance. Compartmentalization
can help to prevent a fire or other systems failure from spreading to
affect other areas of the structure, and may help prevent dangerous
reactions by keeping difference chemicals separate from one another
until they can be safely combined in the proper environment.
Administrative controls
include careful planning and oversight of the refinery cleaning,
maintenance, and turnaround processes. These occur when many of the
engineering controls are shut down or suppressed, and may be especially
dangerous to workers. Detailed coordination is necessary to ensure that
maintenance of one part of the facility will not cause dangerous
exposures to those performing the maintenance, or to workers in other
areas of the plant. Due to the highly flammable nature of many of the
involved chemical, smoking areas are tightly controlled and carefully
placed.
Personal protective equipment
may be necessary depending on the specific chemical being processed or
produced. Particular care is needed during sampling of the
partially-completed product, tank cleaning, and other high-risk tasks as
mentioned above. Such activities may require the use of impervious
outer wear, acid hood, disposable coveralls, etc. More generally, all personnel in operating areas should use appropriate hearing and vision protection, avoid clothes made of flammable material (nylon, Dacron, acrylic, or blends), and full-length pants/sleeves.
Regulations
Worker health and safety in oil refineries is closely monitored by both OSHA and NIOSH. CalOSHA
has been particularly active in regulating worker health in this
industry, and adopted a policy in 2017 that requires petroleum
refineries to perform a Hierarchy of Hazard Controls Analysis (see above
"Controls" section) for each process safety hazard.
Below is a list of the most common regulations referenced in petroleum refinery safety citations issued by OSHA:
- Flammable and Combustible Liquids (29 C.F.R. 1910.106)
- The Hazard Communication (HazCom) standard (29 C.F.R. 1910.1200)
- Permit-Required Confined Spaces (29 C.F.R. 1910.146)
- Hazardous (Classified) Locations (29 C.F.R. 1910.307)
- The Personal Protective Equipment (PPE) standard (29 C.F.R. 1910.132)
- The Control of Hazardous Energy (Lockout/Tagout) standard (29 C.F.R. 1910.147)
Corrosion
Refinery of Slovnaft in Bratislava.
Oil refinery in Iran.
Corrosion of metallic components is a major factor of inefficiency in
the refining process. Because it leads to equipment failure, it is a
primary driver for the refinery maintenance schedule. Corrosion-related
direct costs in the U.S. petroleum industry as of 1996 were estimated at
US $3.7 billion.
Corrosion occurs in various forms in the refining process, such
as pitting corrosion from water droplets, embrittlement from hydrogen,
and stress corrosion cracking from sulfide attack.
From a materials standpoint, carbon steel is used for upwards of 80 per
cent of refinery components, which is beneficial due to its low cost. Carbon steel
is resistant to the most common forms of corrosion, particularly from
hydrocarbon impurities at temperatures below 205 °C, but other corrosive
chemicals and environments prevent its use everywhere. Common
replacement materials are low alloy steels containing chromium and molybdenum, with stainless steels containing more chromium dealing with more corrosive environments. More expensive materials commonly used are nickel, titanium, and copper
alloys. These are primarily saved for the most problematic areas where
extremely high temperatures and/or very corrosive chemicals are present.
Corrosion is fought by a complex system of monitoring,
preventative repairs and careful use of materials. Monitoring methods
include both offline checks taken during maintenance and online
monitoring. Offline checks measure corrosion after it has occurred,
telling the engineer when equipment must be replaced based on the
historical information they have collected. This is referred to as
preventative management.
Online systems are a more modern development, and are
revolutionizing the way corrosion is approached. There are several
types of online corrosion monitoring technologies such as linear
polarization resistance, electrochemical noise
and electrical resistance. Online monitoring has generally had slow
reporting rates in the past (minutes or hours) and been limited by
process conditions and sources of error but newer technologies can
report rates up to twice per minute with much higher accuracy (referred
to as real-time monitoring). This allows process engineers to treat
corrosion as another process variable that can be optimized in the
system. Immediate responses to process changes allow the control of
corrosion mechanisms, so they can be minimized while also maximizing
production output.
In an ideal situation having online corrosion information that is
accurate and real-time will allow conditions that cause high corrosion
rates to be identified and reduced. This is known as predictive
management.
Materials methods include selecting the proper material for the
application. In areas of minimal corrosion, cheap materials are
preferable, but when bad corrosion can occur, more expensive but longer
lasting materials should be used. Other materials methods come in the
form of protective barriers between corrosive substances and the
equipment metals. These can be either a lining of refractory material
such as standard Portland cement
or other special acid-resistant cements that are shot onto the inner
surface of the vessel. Also available are thin overlays of more
expensive metals that protect cheaper metal against corrosion without
requiring lots of material.