An ideal gas is a theoretical gas composed of many randomly moving point particles whose only interactions are perfectly elastic collisions. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics.
In most usual conditions (for instance at standard temperature and pressure), most real gases behave qualitatively like an ideal gas. Many gases such as nitrogen, oxygen, hydrogen, noble gases, and some heavier gases like carbon dioxide can be treated like ideal gases within reasonable tolerances. Generally, a gas behaves more like an ideal gas at higher temperature and lower pressure, as the potential energy due to intermolecular forces becomes less significant compared with the particles' kinetic energy, and the size of the molecules becomes less significant compared to the empty space between them. One mole of an ideal gas has a capacity of 22.710947(13) litres at standard temperature and pressure (a temperature of 273.15 K and an absolute pressure of exactly 105 Pa) as defined by IUPAC since 1982.
The ideal gas model tends to fail at lower temperatures or higher pressures, when intermolecular forces and molecular size becomes important. It also fails for most heavy gases, such as many refrigerants, and for gases with strong intermolecular forces, notably water vapor. At high pressures, the volume of a real gas is often considerably larger than that of an ideal gas. At low temperatures, the pressure of a real gas is often considerably less than that of an ideal gas. At some point of low temperature and high pressure, real gases undergo a phase transition, such as to a liquid or a solid. The model of an ideal gas, however, does not describe or allow phase transitions. These must be modeled by more complex equations of state. The deviation from the ideal gas behaviour can be described by a dimensionless quantity, the compressibility factor, Z.
The ideal gas model has been explored in both the Newtonian dynamics (as in "kinetic theory") and in quantum mechanics (as a "gas in a box"). The ideal gas model has also been used to model the behavior of electrons in a metal (in the Drude model and the free electron model), and it is one of the most important models in statistical mechanics.
Types of ideal gas
There are three basic classes of ideal gas:
- the classical or Maxwell–Boltzmann ideal gas,
- the ideal quantum Bose gas, composed of bosons, and
- the ideal quantum Fermi gas, composed of fermions.
The classical ideal gas can be separated into two types: The
classical thermodynamic ideal gas and the ideal quantum Boltzmann gas.
Both are essentially the same, except that the classical thermodynamic
ideal gas is based on classical statistical mechanics, and certain thermodynamic parameters such as the entropy
are only specified to within an undetermined additive constant. The
ideal quantum Boltzmann gas overcomes this limitation by taking the
limit of the quantum Bose gas and quantum Fermi gas in the limit of high
temperature to specify these additive constants. The behavior of a
quantum Boltzmann gas is the same as that of a classical ideal gas
except for the specification of these constants. The results of the
quantum Boltzmann gas are used in a number of cases including the Sackur–Tetrode equation for the entropy of an ideal gas and the Saha ionization equation for a weakly ionized plasma.
Classical thermodynamic ideal gas
The classical thermodynamic properties of an ideal gas can be described by two equations of state:
Ideal gas law
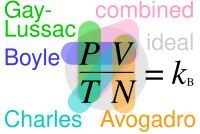
Relationships between Boyle's, Charles's, Gay-Lussac's, Avogadro's, combined and ideal gas laws, with the Boltzmann constant kB = RNA = n RN (in each law, properties circled are constant and properties not circled are variable)
The ideal gas law is the equation of state for an ideal gas, given by:
where
- P is the pressure
- V is the volume
- n is the amount of substance of the gas (in moles)
- R is the gas constant (0.08206 L·atm·K−1·mol−1)
- T is the absolute temperature.
The ideal gas law is an extension of experimentally discovered gas laws. It can also be derived from microscopic considerations.
Real fluids at low density and high temperature
approximate the behavior of a classical ideal gas. However, at lower
temperatures or a higher density, a real fluid deviates strongly from
the behavior of an ideal gas, particularly as it condenses from a gas into a liquid or as it deposits from a gas into a solid. This deviation is expressed as a compressibility factor.
This equation is derived from
- Boyle's law: ;
- Charles's law: ;
- Avogadro's law: .
After combining three laws we get
That is:
- .
Internal energy
The other equation of state of an ideal gas must express Joule's law,
that the internal energy of a fixed mass of ideal gas is a function
only of its temperature. For the present purposes it is convenient to
postulate an exemplary version of this law by writing:
where
- U is the internal energy
- ĉV is the dimensionless specific heat capacity at constant volume, approximately 32 for a monatomic gas, 52 for diatomic gas, and 3 for non-linear molecules if we ignore quantum vibrational contribution. These formulas arise from application of the classical Equipartition Theorem.
That U for an ideal gas depends only on temperature is a consequence of the ideal gas law, although in the general case ĉV depends on temperature and an integral is needed to compute U.
Microscopic model
In
order to switch from macroscopic quantities (left hand side of the
following equation) to microscopic ones (right hand side), we use
where
- N is the number of gas particles
- kB is the Boltzmann constant (1.381×10−23 J·K−1).
The probability distribution of particles by velocity or energy is given by the Maxwell speed distribution.
The ideal gas model depends on the following assumptions:
- The molecules of the gas are indistinguishable, small, hard spheres
- All collisions are elastic and all motion is frictionless (no energy loss in motion or collision)
- Newton's laws apply
- The average distance between molecules is much larger than the size of the molecules
- The molecules are constantly moving in random directions with a distribution of speeds
- There are no attractive or repulsive forces between the molecules apart from those that determine their point-like collisions
- The only forces between the gas molecules and the surroundings are those that determine the point-like collisions of the molecules with the walls
- In the simplest case, there are no long-range forces between the molecules of the gas and the surroundings.
The assumption of spherical particles is necessary so that there are
no rotational modes allowed, unlike in a diatomic gas. The following
three assumptions are very related: molecules are hard, collisions are
elastic, and there are no inter-molecular forces. The assumption that
the space between particles is much larger than the particles themselves
is of paramount importance, and explains why the ideal gas
approximation fails at high pressures.
Heat capacity
The dimensionless heat capacity at constant volume is generally defined by
where S is the entropy.
This quantity is generally a function of temperature due to
intermolecular and intramolecular forces, but for moderate temperatures
it is approximately constant. Specifically, the Equipartition Theorem predicts that the constant for a monatomic gas is ĉV = 32 while for a diatomic gas it is ĉV = 52
if vibrations are neglected (which is often an excellent
approximation). Since the heat capacity depends on the atomic or
molecular nature of the gas, macroscopic measurements on heat capacity
provide useful information on the microscopic structure of the
molecules.
The dimensionless heat capacity at constant pressure of an ideal gas is:
where H = U + PV is the enthalpy of the gas.
Sometimes, a distinction is made between an ideal gas, where ĉV and ĉP could vary with temperature, and a perfect gas, for which this is not the case.
The ratio of the constant volume and constant pressure heat capacity is the adiabatic index
For air, which is a mixture of gases, this ratio is 1.4.
Entropy
Using the results of thermodynamics only, we can go a long way in determining the expression for the entropy of an ideal gas. This is an important step since, according to the theory of thermodynamic potentials, if we can express the entropy as a function of U (U is a thermodynamic potential), volume V and the number of particles N,
then we will have a complete statement of the thermodynamic behavior of
the ideal gas. We will be able to derive both the ideal gas law and the
expression for internal energy from it.
Since the entropy is an exact differential, using the chain rule, the change in entropy when going from a reference state 0 to some other state with entropy S may be written as ΔS where:
where the reference variables may be functions of the number of particles N. Using the definition of the heat capacity at constant volume for the first differential and the appropriate Maxwell relation for the second we have:
Expressing CV in terms of ĉV as developed in the above section, differentiating the ideal gas equation of state, and integrating yields:
which implies that the entropy may be expressed as:
where all constants have been incorporated into the logarithm as f(N) which is some function of the particle number N having the same dimensions as VTĉV
in order that the argument of the logarithm be dimensionless. We now
impose the constraint that the entropy be extensive. This will mean that
when the extensive parameters (V and N) are multiplied by a constant, the entropy will be multiplied by the same constant. Mathematically:
From this we find an equation for the function f(N)
Differentiating this with respect to a, setting a equal to 1, and then solving the differential equation yields f(N):
where Φ may vary for different gases, but will be independent of the thermodynamic state of the gas. It will have the dimensions of VTĉV/N. Substituting into the equation for the entropy:
and using the expression for the internal energy of an ideal gas, the entropy may be written:
Since this is an expression for entropy in terms of U, V, and N, it is a fundamental equation from which all other properties of the ideal gas may be derived.
This is about as far as we can go using thermodynamics alone.
Note that the above equation is flawed – as the temperature approaches
zero, the entropy approaches negative infinity, in contradiction to the third law of thermodynamics.
In the above "ideal" development, there is a critical point, not at
absolute zero, at which the argument of the logarithm becomes unity, and
the entropy becomes zero. This is unphysical. The above equation is a
good approximation only when the argument of the logarithm is much
larger than unity – the concept of an ideal gas breaks down at low
values of VN.
Nevertheless, there will be a "best" value of the constant in the sense
that the predicted entropy is as close as possible to the actual
entropy, given the flawed assumption of ideality. A quantum-mechanical
derivation of this constant is developed in the derivation of the Sackur–Tetrode equation which expresses the entropy of a monatomic (ĉV = 32)
ideal gas. In the Sackur–Tetrode theory the constant depends only upon
the mass of the gas particle. The Sackur–Tetrode equation also suffers
from a divergent entropy at absolute zero, but is a good approximation
for the entropy of a monatomic ideal gas for high enough temperatures.



















![{\displaystyle {\frac {S}{Nk}}=\ln \left[{\frac {V}{N}}\,\left({\frac {U}{{\hat {c}}_{V}kN}}\right)^{{\hat {c}}_{V}}\,{\frac {1}{\Phi }}\right]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/65d5055ff166a2ab540fb032ec1d0ace534b5966)
