 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˈklɔːrəkwɪn/ |
| Trade names | Aralen, others |
| AHFS/Drugs.com | Monograph |
| License data |
|
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Liver |
| Elimination half-life | 1–2 months |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.175 |
| Chemical and physical data | |
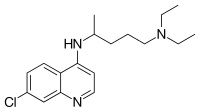
| Formula | C18H26ClN3 |
| Molar mass | 319.872 g·mol−1 |
| 3D model (JSmol) | |
Chloroquine is a medication used to prevent and to treat malaria in areas where malaria is known to be sensitive to its effects. Certain types of malaria, resistant strains, and complicated cases typically require different or additional medication. Occasionally it is used for amebiasis that is occurring outside the intestines, rheumatoid arthritis, and lupus erythematosus. It is taken by mouth. It is also being used experimentally to treat COVID-19 as of 2020.
Common side effects include muscle problems, loss of appetite, diarrhea, and skin rash. Serious side effects include problems with vision, muscle damage, seizures, and low blood cell levels. It appears to be safe for use during pregnancy. Chloroquine is a member of the drug class 4-aminoquinoline. It works against the asexual form of malaria inside the red blood cell.
Chloroquine was discovered in 1934 by Hans Andersag. It is on the World Health Organization's List of Essential Medicines, the medicines needed in a health system that are considered to be the safest and most effective. It is available as a generic medication. The wholesale cost in the developing world is about US$0.04. In the United States, it costs about US$5.30 per dose.
Medical uses
Resochin tablet package
Malaria
Chloroquine has long been used in the treatment or prevention of malaria from Plasmodium vivax, P. ovale, and P. malariae, excluding the malaria parasite Plasmodium falciparum, for it started to develop widespread resistance to it.
Chloroquine has been extensively used in mass drug administrations,
which may have contributed to the emergence and spread of resistance.
It is recommended to check if chloroquine is still effective in the
region prior to using it. In areas where resistance is present, other antimalarials, such as mefloquine or atovaquone, may be used instead. The Centers for Disease Control and Prevention recommend against treatment of malaria with chloroquine alone due to more effective combinations.
Amebiasis
In treatment of amoebic liver abscess, chloroquine may be used instead of or in addition to other medications in the event of failure of improvement with metronidazole or another nitroimidazole within 5 days or intolerance to metronidazole or a nitroimidazole.
Rheumatic disease
As it mildly suppresses the immune system, chloroquine is used in some autoimmune disorders, such as rheumatoid arthritis and lupus erythematosus.
Side effects
Side effects
include blurred vision, nausea, vomiting, abdominal cramps, headache,
diarrhea, swelling legs/ankles, shortness of breath, pale
lips/nails/skin, muscle weakness, easy bruising/bleeding, hearing and
mental problems. Other effects might include cardiovascular (rare), and blood reactions.
- Seizures
- Unwanted/uncontrolled movements (including tongue and face twitching)
- Deafness or tinnitus.
- Nausea, vomiting, diarrhea, abdominal cramps, and anorexia
- Headache.
- Mental/mood changes (such as confusion, personality changes, unusual thoughts/behavior, depression, feeling being watched, hallucinating)
- Signs of serious infection (such as high fever, severe chills, persistent sore throat)
- Skin itchiness, skin color changes, hair loss, and skin rashes.
- Chloroquine-induced itching is very common among black Africans (70%), but much less common in other races. It increases with age, and is so severe as to stop compliance with drug therapy. It is increased during malaria fever; its severity is correlated to the malaria parasite load in blood. Some evidence indicates it has a genetic basis and is related to chloroquine action with opiate receptors centrally or peripherally.
- Unpleasant metallic taste
- This could be avoided by "taste-masked and controlled release" formulations such as multiple emulsions.
- Chloroquine retinopathy
- May be irreversible. This occurs with long-term use over many years or with high doses. Patients on long-term chloroquine therapy should be screened at baseline and then annually after five years of use. Patients should be screened for vision changes such as blurring of vision, difficulty focusing, or seeing half an object.
- Hypotension and electrocardiographic changes.
- This manifests itself as either conduction disturbances (bundle-branch block, atrioventricular block) or Cardiomyopathy – often with hypertrophy, restrictive physiology, and congestive heart failure. The changes may be irreversible. Only two cases have been reported requiring heart transplantation, suggesting this particular risk is very low. Electron microscopy of cardiac biopsies show pathognomonic cytoplasmic inclusion bodies.
- Pancytopenia, aplastic anemia, reversible agranulocytosis, low blood platelets, neutropenia.
Pregnancy
Chloroquine has not been shown to have any harmful effects on the fetus when used for malarial prophylaxis.
Small amounts of chloroquine are excreted in the breast milk of
lactating women. However, this drug can be safely prescribed to infants,
the effects are not harmful. Studies with mice show that radioactively
tagged chloroquine passed through the placenta rapidly and accumulated
in the fetal eyes which remained present five months after the drug was
cleared from the rest of the body. Women who are pregnant or planning on getting pregnant are still advised against traveling to malaria-risk regions.
Elderly
There
is not enough evidence to determine whether chloroquine is safe to be
given to people aged 65 and older. However, the drug is cleared by the
kidneys and toxicity should be monitored carefully in people with poor
kidney functions.
Drug interactions
- Ampicillin- levels may be reduced by chloroquine
- Antacids- may reduce absorption of chloroquine
- Cimetidine- may inhibit metabolism of chloroquine; increasing levels of chloroquine in the body
- Cyclosporine- levels may be increased by chloroquine
- Kaolin- may reduce absorption of chloroquine
- Mefloquine- may increase risk of convulsions
Overdose
Chloroquine
is very dangerous in overdose. It is rapidly absorbed from the gut. In
1961, a published compilation of case reports contained accounts of
three children who took overdoses and died within 2.5 hours of taking
the drug. While the amount of the overdose was not stated, the therapeutic index for chloroquine is known to be small.
Symptoms of overdose include headache, drowsiness, visual disturbances,
nausea and vomiting, cardiovascular collapse, seizures, and sudden
respiratory and cardiac arrest.
An analog of chloroquine – hydroxychloroquine – has a long half-life (32–56 days) in blood and a large volume of distribution (580–815 L/kg).
The therapeutic, toxic and lethal ranges are usually considered to be
0.03 to 15 mg/l, 3.0 to 26 mg/l and 20 to 104 mg/l, respectively.
However, nontoxic cases have been reported up to 39 mg/l, suggesting
individual tolerance to this agent may be more variable than previously
recognised.
Pharmacology
- Absorption: Rapid and almost completely
- Distribution: Widely distributed into body tissues
- Protein binding: 55%
- Metabolism: Partially hepatic to main metabolite, desethylchloroquine
- Excretion: Urine (≥50% as unchanged drug); acidification of urine increases elimination
Chloroquine has a very high volume of distribution, as it diffuses into the body's adipose tissue. Chloroquine and related quinines have been associated with cases of retinal
toxicity, particularly when provided at higher doses for longer times.
Accumulation of the drug may result in deposits that can lead to blurred
vision and blindness. With long-term doses, routine visits to an ophthalmologist are recommended.
Chloroquine is also a lysosomotropic agent, meaning it accumulates preferentially in the lysosomes of cells in the body. The pKa
for the quinoline nitrogen of chloroquine is 8.5, meaning it is about
10% deprotonated at physiological pH as calculated by the Henderson-Hasselbalch equation.
This decreases to about 0.2% at a lysosomal pH of 4.6. Because the
deprotonated form is more membrane-permeable than the protonated form, a
quantitative "trapping" of the compound in lysosomes results. (A
quantitative treatment of this phenomenon involves the pKas of all nitrogens in the molecule; this treatment, however, suffices to show the principle.)
The lysosomotropic character of chloroquine is believed to
account for much of its antimalarial activity; the drug concentrates in
the acidic food vacuole of the parasite and interferes with essential
processes. Its lysosomotropic properties further allow for its use for in vitro experiments pertaining to intracellular lipid related diseases, autophagy, and apoptosis.
Mechanism of action
Medical quinolines
Malaria
Hemozoin formation in P. falciparum: many antimalarials are strong inhibitors of hemozoin crystal growth.
Inside red blood cells, the malarial parasite, which is then in its asexual lifecycle stage, must degrade hemoglobin to acquire essential amino acids, which the parasite requires to construct its own protein and for energy metabolism. Digestion is carried out in a vacuole of the parasitic cell.
Hemoglobin is composed of a protein unit (digested by the
parasite) and a heme unit (not used by the parasite). During this
process, the parasite releases the toxic and soluble molecule heme.
The heme moiety consists of a porphyrin ring called
Fe(II)-protoporphyrin IX (FP). To avoid destruction by this molecule,
the parasite biocrystallizes heme to form hemozoin, a nontoxic molecule. Hemozoin collects in the digestive vacuole as insoluble crystals.
Chloroquine enters the red blood cell by simple diffusion,
inhibiting the parasite cell and digestive vacuole. Chloroquine then
becomes protonated (to CQ2+), as the digestive vacuole is known to be
acidic (pH 4.7); chloroquine then cannot leave by diffusion. Chloroquine
caps hemozoin molecules to prevent further biocrystallization
of heme, thus leading to heme buildup. Chloroquine binds to heme (or
FP) to form the FP-chloroquine complex; this complex is highly toxic to
the cell and disrupts membrane function. Action of the toxic
FP-chloroquine and FP results in cell lysis and ultimately parasite cell
autodigestion. Parasites that do not form hemozoin are therefore resistant to chloroquine.
Resistance in malaria
Since the first documentation of P. falciparum
chloroquine resistance in the 1950s, resistant strains have appeared
throughout East and West Africa, Southeast Asia, and South America. The
effectiveness of chloroquine against P. falciparum has declined
as resistant strains of the parasite evolved. They effectively
neutralize the drug via a mechanism that drains chloroquine away from
the digestive vacuole. Chloroquine-resistant cells efflux chloroquine at
40 times the rate of chloroquine-sensitive cells; the related mutations
trace back to transmembrane proteins of the digestive vacuole,
including sets of critical mutations in the P. falciparum chloroquine resistance transporter (PfCRT) gene. The mutated protein, but not the wild-type transporter, transports chloroquine when expressed in Xenopus oocytes (frog's eggs) and is thought to mediate chloroquine leak from its site of action in the digestive vacuole. Resistant parasites also frequently have mutated products of the ABC transporter P. falciparum multidrug resistance (PfMDR1) gene, although these mutations are thought to be of secondary importance compared to Pfcrt. Verapamil, a Ca2+
channel blocker, has been found to restore both the chloroquine
concentration ability and sensitivity to this drug. Recently, an altered
chloroquine-transporter protein CG2 of the parasite has been related to
chloroquine resistance, but other mechanisms of resistance also appear
to be involved.
Research on the mechanism of chloroquine and how the parasite has
acquired chloroquine resistance is still ongoing, as other mechanisms of
resistance are likely.
Other agents which have been shown to reverse chloroquine resistance in malaria are chlorpheniramine, gefitinib, imatinib, tariquidar and zosuquidar.
Antiviral
Chloroquine has antiviral effects. It increases endosomal pH, resulting in impaired virus/cell fusion - fusion requires a low pH.
Chloroquine also seems to act as a zinc ionophore, thereby
allowing extra cellular zinc to enter inside the cell and inhibit viral
RNA dependant RNA polymerase.
Other
Against rheumatoid arthritis, it operates by inhibiting lymphocyte proliferation, phospholipase A2, antigen presentation in dendritic cells, release of enzymes from lysosomes, release of reactive oxygen species from macrophages, and production of IL-1.
History
In Peru the indigenous people extracted the bark of the Cinchona plant trees and used the extract (Chinchona officinalis)
to fight chills and fever in the seventeenth century. In 1633 this
herbal medicine was introduced in Europe, where it was given the same
use and also began to be used against malaria. The quinoline antimalarial drug quinine was isolated from the extract in 1820, and chloroquine is an analogue of this.
Chloroquine was discovered in 1934 by Hans Andersag and coworkers at the Bayer laboratories, who named it "Resochin".
It was ignored for a decade, because it was considered too toxic for
human use. During World War II, United States government-sponsored
clinical trials for antimalarial drug development showed unequivocally
that chloroquine has a significant therapeutic value as an antimalarial
drug. It was introduced into clinical practice in 1947 for the
prophylactic treatment of malaria.
Names
Brand names include Chloroquine FNA, Resochin, Dawaquin, and Lariago.
Research
COVID-19
In late January 2020 during the 2019–20 coronavirus outbreak, Chinese medical researchers stated that exploratory research into chloroquine and two other medications, remdesivir and lopinavir/ritonavir, seemed to have "fairly good inhibitory effects" on the SARS-CoV-2 virus, which is the virus that causes COVID-19. Requests to start clinical testing were submitted.
Chloroquine had been also proposed as a treatment for SARS, with in vitro tests inhibiting the SARS-CoV virus.
On 19 February 2020, preliminary results found that chloroquine
may be effective and safe in treating COVID-19 associated pneumonia.
The Guangdong Provincial Department of Science and Technology and the
Guangdong Provincial Health and Health Commission issued a report
stating that chloroquine phosphate "improves the success rate of
treatment and shortens the length of patient’s hospital stay" and
recommended it for people diagnosed with mild, moderate and severe cases
of novel coronavirus pneumonia.
Chloroquine has been recommended by Chinese, South Korean and Italian health authorities for the treatment of COVID-19. These agencies noted contraindications for people with heart disease or diabetes. In February 2020, both drugs were shown to effectively inhibit COVID-19 in vitro, but a further study concluded that hydroxychloroquine was more potent than chloroquine, with a more tolerable safety profile.
Preliminary results from a trial suggested that chloroquine is
effective and safe in COVID-19 pneumonia, "improving lung imaging
findings, promoting a virus-negative conversion, and shortening the
disease course."
Other viruses
In
October 2004 a group of researchers at the Rega Institute for Medical
Research published a report on chloroquine, stating that chloroquine
acts as an effective inhibitor of the replication of the severe acute
respiratory syndrome coronavirus (SARS-CoV) in vitro.
Chloroquine is being considered in pre-clinical models as a potential agent against chikungunya fever.
Other
The radiosensitizing and chemosensitizing properties of chloroquine are beginning to be exploited in anticancer strategies in humans. In biomedicinal science, chloroquine is used for in vitro experiments to inhibit lysosomal degradation of protein products.