 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| MedlinePlus | a682860 |
| Pregnancy category |
|
| Routes of administration | IV |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 100% (IV) |
| Protein binding | 50% |
| Elimination half-life | 1–2 hours |
| Identifiers | |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
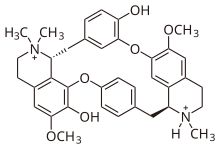
| Formula | C37H42Cl2N2O6 |
| Molar mass | 681.65 g/mol g·mol−1 |
| 3D model (JSmol) | |
Tubocurarine (also known as d-tubocurarine or DTC) is a toxic alkaloid historically known for its use as an arrow poison. In the mid-1900s, it was used in conjunction with an anesthetic to provide skeletal muscle relaxation during surgery or mechanical ventilation. It is now rarely used as an adjunct for clinical anesthesia because safer alternatives, such as cisatracurium and rocuronium, are available.
History
Tubocurarine is a naturally occurring mono-quaternary alkaloid obtained from the bark of the Menispermaceous South American plant Chondrodendron tomentosum, a climbing vine known to the European world since the Spanish conquest of South America. Curare had been used as a source of arrow poison
by South American natives to hunt animals, and they were able to eat
the animals' contaminated flesh subsequently without any adverse effects
because tubocurarine cannot easily cross mucous membranes.Thus, tubocurarine is effective only if given parenterally, as demonstrated by Bernard, who also showed that the site of its action was at the neuromuscular junction. Virchow and Munter confirmed the paralyzing action was limited to voluntary muscles.
Etymology
The word "curare" comes from the South American Native name for the arrow poison, ourare. Presumably, the initial syllable was pronounced with a heavy glottal stop.
Tubocurarine is so-called because some of the plant extracts designated
'curare' were stored, and subsequently shipped to Europe, in bamboo
tubes. Likewise, curare stored in calabash containers was called calabash curare, although this was usually an extract not of Chondrodendron, but of the Strychnos species S. toxifera, containing a different alkaloid, namely toxiferine. Pot curare was generally a mixture of extracts from various genera in the families Menispermaceae and Strychnaceae.
The tripartite classification into 'tube' , 'calabash' and 'pot'
curares early became untenable, due to inconsistencies in the use of the
different types of vessels and the complexities of the dart poison
recipes themselves.
Use as an anesthetic
Griffith
and Johnson are credited with pioneering the formal clinical
introduction of tubocurarine as an adjunct to anesthetic practice on 23
January 1942, at the Montreal Homeopathic Hospital. In this sense, tubocurarine is the prototypical adjunctive neuromuscular non-depolarizing agent. However, others before Griffith and Johnson had attempted use of tubocurarine in several situations: some under controlled study conditions while others not quite controlled and remained unpublished.
Regardless, all in all some 30,000 patients had been given tubocurarine
by 1941, although it was Griffith and Johnson's 1942 publication
that provided the impetus to the standard use of neuromuscular blocking
agents in clinical anesthestic practice – a revolution that rapidly
metamorphosized into the standard practice of "balanced" anesthesia: the
triad of barbiturate hypnosis, light inhalational anesthesia and muscle
relaxation. The technique as described by Gray and Halton was widely known as the "Liverpool technique",
and became the standard anesthetic technique in England in the 1950s
and 1960s for patients of all ages and physical status. Present clinical
anesthetic practice still employs the central principle of balanced
anesthesia though with some differences to accommodate subsequent
technological advances and introductions of new and better gaseous
anesthetic, hypnotic and neuromuscular blocking agents, and tracheal
intubation, as well as monitoring techniques that were nonexistent in
the day of Gray and Halton: pulse oximetry, capnography, peripheral
nerve stimulation, noninvasive blood pressure monitoring, etc.
Chemical properties
Structurally, tubocurarine is a benzylisoquinoline derivative. For many years, its structure, when first elucidated in 1948, was wrongly thought to be bis-quaternary: in other words, it was thought to be an N,N-dimethylated alkaloid. In 1970, the correct structure was finally established, showing one of the two nitrogens to be tertiary, actually a mono-N-methylated alkaloid.
Biosynthesis
Tubocurarine biosynthesis involves a radical coupling of the two enantiomers of N-methylcoclaurine. (R) and (S)-N-methylcoclaurine
come from a Mannich-like reaction between dopamine and
4-hydroxyphenylacetaldehyde, facilitated by norcoclaurine synthase
(NCS). Both dopamine and 4-hydroxyphenylacetaldehyde originate from L-tyrosine. Methylation of the amine and hydroxyl substituents are facilitated by S-adenosyl methionine (SAM).
One methyl group is present on each nitrogen atom prior to the radical
coupling. The additional methyl group is transferred to form
tubocurarine, with its single quaternary N,N-dimethylamino group.
Biological effects
Acetylcholine in the synaptic gap
Without intervention, acetylcholine (ACh) in the peripheral nervous system activates skeletal muscles. Acetylcholine is produced in the body of the neuron by choline acetyltransferase and transported down the axon to the synaptic gap. Tubocurarine chloride acts as an antagonist for the nicotinic acetylcholine receptor (nAChr), meaning it blocks the receptor site from ACh. This may be due to the quaternary amino structural motif found on both molecules.
Clinical pharmacology
Unna et al. reported the effects of tubocurarine on humans:
"Forty-five seconds after the beginning of the injection, heaviness of the eyelids and transitory diplopia
were perceived. At the completion of the injection, diplopia became
fixed, but could be noticed only when the subject’s eyelids were raised
by the operator. As curarization proceeded, it seemed to the subject as
if the facial muscles, those of the tongue, pharynx, and lower jaw, the
muscles of the neck and back, and the muscles of the extremities became
relaxed in about that order. Accompanying the paralysis of the pharynx
and the jaw muscles, inability of the subject to swallow was noted …
Shortly after the injection was completed the subjects experienced a
sensation of increased difficulty in breathing, as if an extra effort
was necessary to maintain an adequate respiratory exchange. This
sensation was present even though there was no objective evidence of
impaired oxygenation or of carbon dioxide retention. It reached its
maximum about five minutes after the injection, coinciding with the
maximum depression of the vital capacity. In the majority of the
experiments the respiratory rate was increased by about 50–100 per cent
the first minutes after the injection of any one of the drugs while the
tidal volume decreased."
Tubocurarine has a time of onset of around 5 minutes which is relatively slow among neuromuscular-blocking drugs, and has a duration of action of 60 to 120 minutes. It also causes histamine release,
now a recognized hallmark of the tetrahydroisioquinolinium class of
neuromuscular blocking agents. Histamine release is associated with bronchospasms, hypotension, salivary secretions, making it dangerous for asthmatics, children, and those who are pregnant or lactating. However, the main disadvantage in the use of tubocurarine is its significant ganglion-blocking effect, that manifests as hypotension, in many patients; this constitutes a relative contraindication to its use in patients with myocardial ischaemia.
Because of the shortcomings of tubocurare, much research effort
was undertaken soon after its clinical introduction to find a suitable
replacement. The efforts unleashed a multitude of compounds borne from
structure-activity relations developed from the tubocurare molecule.
Some key compounds that have seen clinical use are identified in the
muscle relaxants template box below. Of the many tried as replacements,
only a few enjoyed as much popularity as tubocurarine: pancuronium, vecuronium, rocuronium, atracurium, and cisatracurium. Succinylcholine is a widely used muscle relaxant drug which acts by activating, instead of blocking, the ACh receptor.
The potassium channel blocker tetraethylammonium
(TEA) has been shown to reverse the effects of tubocurarine. It is
thought to do so by increasing ACh release, which counteracts the
antagonistic effects of tubocurarine on the ACh receptor.
Use as spider bite treatment
Spiders of the genus Latrodectus have α-latrotoxin
in their venom. The most well known spider in this genus is the black
widow spider. α-latrotoxin causes the release of neurotransmitters into
the synaptic gap, including acetylcholine.
Bites are usually not fatal, but do cause a significant amount of pain
in addition to muscle spasms. The venom is the most damaging to nerve
endings, but the introduction of d-tubocurarine chloride blocks the nAChr, alleviating pain and muscle spasms while an antivenom can be administered.
Toxicology
An individual administered tubocurarine chloride will be unable to move any voluntary muscles, including the diaphragm. A large enough dose will therefore result in death from respiratory failure unless artificial ventilation is initiated. The LD50 for mice and rabbits are 0.13 mg/kg and 0.146 mg/kg intravenously, respectively. It releases histamine and causes hypotension.


