| Glucocorticoid | |
|---|---|
| Drug class | |

Chemical structure of cortisol (hydrocortisone), an endogenous glucocorticoid as well as medication.
| |
| Class identifiers | |
| Synonyms | Corticosteroid; Glucocorticosteroid |
| Use | Adrenal insufficiency; allergic, inflammatory, and autoimmune disorders; asthma; organ transplant |
| ATC code | H02AB |
| Biological target | Glucocorticoid receptor |
| Chemical class | Steroids |
Glucocorticoids are a class of corticosteroids, which are a class of steroid hormones. Glucocorticoids are corticosteroids that bind to the glucocorticoid receptor that is present in almost every vertebrate animal cell. The name "glucocorticoid" is a portmanteau (glucose + cortex + steroid) and is composed from its role in regulation of glucose metabolism, synthesis in the adrenal cortex, and its steroidal structure (see structure to the right). A less common synonym is glucocorticosteroid.
Glucocorticoids are part of the feedback mechanism in the immune system which reduces certain aspects of immune function, such as inflammation. They are therefore used in medicine to treat diseases caused by an overactive immune system, such as allergies, asthma, autoimmune diseases, and sepsis. Glucocorticoids have many diverse (pleiotropic) effects, including potentially harmful side effects, and as a result are rarely sold over the counter. They also interfere with some of the abnormal mechanisms in cancer cells, so they are used in high doses to treat cancer. This includes inhibitory effects on lymphocyte proliferation, as in the treatment of lymphomas and leukemias, and the mitigation of side effects of anticancer drugs.
Glucocorticoids affect cells by binding to the glucocorticoid receptor. The activated glucocorticoid receptor-glucocorticoid complex up-regulates the expression of anti-inflammatory proteins in the nucleus (a process known as transactivation) and represses the expression of proinflammatory proteins in the cytosol by preventing the translocation of other transcription factors from the cytosol into the nucleus (transrepression).
Glucocorticoids are distinguished from mineralocorticoids and sex steroids by their specific receptors, target cells, and effects. In technical terms, "corticosteroid" refers to both glucocorticoids and mineralocorticoids (as both are mimics of hormones produced by the adrenal cortex), but is often used as a synonym for "glucocorticoid". Glucocorticoids are chiefly produced in the zona fasciculata of the adrenal cortex, whereas mineralocorticoids are synthesized in the zona glomerulosa.
Cortisol (or hydrocortisone) is the most important human glucocorticoid. It is essential for life, and it regulates or supports a variety of important cardiovascular, metabolic, immunologic, and homeostatic functions. Various synthetic glucocorticoids are available; these are widely utilized in general medical practice and numerous specialties either as replacement therapy in glucocorticoid deficiency or to suppress the immune system.
Effects
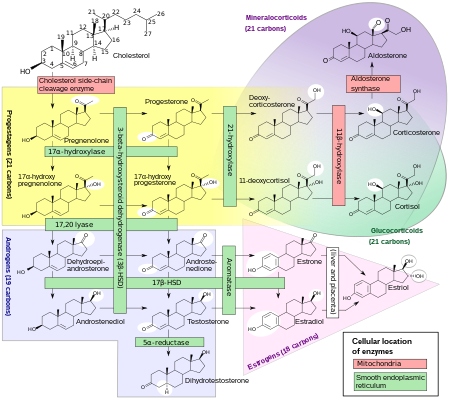
Steroidogenesis showing glucocorticoids in green ellipse at right with the primary example being cortisol.[3] It is not a strictly bounded group, but a continuum of structures with increasing glucocorticoid effect.
Glucocorticoid effects may be broadly classified into two major categories: immunological and metabolic. In addition, glucocorticoids play important roles in fetal development and body fluid homeostasis.
Immune
As discussed in more detail below, glucocorticoids function through interaction with the glucocorticoid receptor:
- up-regulate the expression of anti-inflammatory proteins.
- down-regulate the expression of proinflammatory proteins.
Glucocorticoids are also shown to play a role in the development and
homeostasis of T lymphocytes. This has been shown in transgenic mice
with either increased or decreased sensitivity of T cell lineage to
glucocorticoids.
Metabolic
The name "glucocorticoid" derives from early observations that these hormones were involved in glucose metabolism. In the fasted state, cortisol stimulates several processes that collectively serve to increase and maintain normal concentrations of glucose in blood.
Metabolic effects:
- Stimulation of gluconeogenesis, in particular, in the liver: This pathway results in the synthesis of glucose from non-hexose substrates, such as amino acids and glycerol from triglyceride breakdown, and is particularly important in carnivores and certain herbivores. Enhancing the expression of enzymes involved in gluconeogenesis is probably the best-known metabolic function of glucocorticoids.
- Mobilization of amino acids from extrahepatic tissues: These serve as substrates for gluconeogenesis.
- Inhibition of glucose uptake in muscle and adipose tissue: A mechanism to conserve glucose
- Stimulation of fat breakdown in adipose tissue: The fatty acids released by lipolysis are used for production of energy in tissues like muscle, and the released glycerol provide another substrate for gluconeogenesis.
- Increase in sodium retention and potassium excretion leads to hypernatremia and hypokalemia
- Increase in hemoglobin concentration, likely due to hindrance of the ingestion of red blood cell by macrophage or other phagocyte.
- Increased urinary uric acid
- Increased urinary calcium and hypocalcemia
- Alkalosis
- Leukocytosis
Excessive glucocorticoid levels resulting from administration as a drug or hyperadrenocorticism
have effects on many systems. Some examples include inhibition of bone
formation, suppression of calcium absorption (both of which can lead to osteoporosis),
delayed wound healing, muscle weakness, and increased risk of
infection. These observations suggest a multitude of less-dramatic
physiologic roles for glucocorticoids.
Developmental
Glucocorticoids
have multiple effects on fetal development. An important example is
their role in promoting maturation of the lung and production of the surfactant necessary for extrauterine lung function. Mice with homozygous disruptions in the corticotropin-releasing
hormone gene (see below) die at birth due to pulmonary immaturity. In
addition, glucocorticoids are necessary for normal brain development, by
initiating terminal maturation, remodeling axons and dendrites, and
affecting cell survival and may also play a role in hippocampal development. Glucocorticoids stimulate the maturation of the Na+/K+/ATPase,
nutrient transporters, and digestion enzymes, promoting the development
of a functioning gastro-intestinal system. Glucocorticoids also support
the development of the neonate's renal system by increasing glomerular
filtration.
Arousal and cognition
A graphical representation of the Yerkes-Dodson curve
Glucocorticoids act on the hippocampus, amygdala, and frontal lobes. Along with adrenaline, these enhance the formation of flashbulb memories of events associated with strong emotions, both positive and negative.
This has been confirmed in studies, whereby blockade of either
glucocorticoids or noradrenaline activity impaired the recall of
emotionally relevant information. Additional sources have shown subjects
whose fear learning was accompanied by high cortisol levels had better
consolidation of this memory (this effect was more important in men).
The effect that glucocorticoids have on memory may be due to damage
specifically to the CA1 area of the hippocampal formation. In multiple
animal studies, prolonged stress (causing prolonged increases in
glucocorticoid levels) have shown destruction of the neurons in this
area of the brain, which has been connected to lower memory performance.
Glucocorticoids have also been shown to have a significant impact on vigilance (attention deficit disorder) and cognition (memory). This appears to follow the Yerkes-Dodson curve,
as studies have shown circulating levels of glucocorticoids vs. memory
performance follow an upside-down U pattern, much like the Yerkes-Dodson
curve. For example, long-term potentiation
(LTP; the process of forming long-term memories) is optimal when
glucocorticoid levels are mildly elevated, whereas significant decreases
of LTP are observed after adrenalectomy (low-glucocorticoid state) or
after exogenous glucocorticoid administration (high-glucocorticoid
state). Elevated levels of glucocorticoids enhance memory for
emotionally arousing events, but lead more often than not to poor memory
for material unrelated to the source of stress/emotional arousal.
In contrast to the dose-dependent enhancing effects of glucocorticoids
on memory consolidation, these stress hormones have been shown to
inhibit the retrieval of already stored information.
Long-term exposure to glucocorticoid medications, such as asthma and
anti-inflammatory medication, has been shown to create deficits in
memory and attention both during and, to a lesser extent, after
treatment, a condition known as "steroid dementia".
Body fluid homeostasis
Glucocorticoids
could act centrally, as well as peripherally, to assist in the
normalization of extracellular fluid volume by regulating body's action
to atrial natriuretic peptide (ANP). Centrally, glucocorticoids could inhibit dehydration induced water intake; peripherally, glucocorticoids could induce a potent diuresis.
Mechanism of action
Transactivation
Glucocorticoids bind to the cytosolic glucocorticoid receptor, a type of nuclear receptor that is activated by ligand binding. After a hormone binds to the corresponding receptor, the newly formed complex translocates itself into the cell nucleus, where it binds to glucocorticoid response elements in the promoter region of the target genes resulting in the regulation of gene expression. This process is commonly referred to as transcriptional activation, or transactivation.
The proteins encoded by these up-regulated genes have a wide range of effects, including, for example:
- anti-inflammatory – lipocortin I, p11/calpactin binding protein, secretory leukocyte protease inhibitor 1 (SLPI), and Mitogen-activated protein kinase phosphatase (MAPK phosphatase)
- increased gluconeogenesis – glucose 6-phosphatase and tyrosine aminotransferase
Transrepression
The opposite mechanism is called transcriptional repression, or transrepression.
The classical understanding of this mechanism is that activated
glucocorticoid receptor binds to DNA in the same site where another transcription factor would bind, which prevents the transcription of genes that are transcribed via the activity of that factor.
While this does occur, the results are not consistent for all cell
types and conditions; there is no generally accepted, general mechanism
for transrepression.
New mechanisms are being discovered where transcription is
repressed, but the activated glucocorticoid receptor is not interacting
with DNA, but rather with another transcription factor directly, thus
interfering with it, or with other proteins that interfere with the
function of other transcription factors. This latter mechanism appears
to be the most likely way that activated glucocorticoid receptor
interferes with NF-κB - namely by recruiting histone deacetylase, which deacetylate the DNA in the promoter region leading to closing of the chromatin structure where NF-κB needs to bind.
Nongenomic effects
Activated
glucocorticoid receptor has effects that have been experimentally shown
to be independent of any effects on transcription and can only be due
to direct binding of activated glucocorticoid receptor with other
proteins or with mRNA.
For example, Src kinase
which binds to inactive glucocorticoid receptor, is released when a
glucocorticoid binds to glucocorticoid receptor, and phosphorylates a
protein that in turn displaces an adaptor protein from a receptor
important in inflammation, epidermal growth factor, reducing its activity, which in turn results in reduced creation of arachidonic acid - a key proinflammatory molecule. This is one mechanism by which glucocorticoids have an anti-inflammatory effect.
Pharmacology
Dexamethasone - a synthetic glucocorticoid binds more powerfully to the glucocorticoid receptor
than cortisol does. Dexamethasone is based on the cortisol structure
but differs at three positions (extra double bond in the A-ring between
carbons 1 and 2 and addition of a 9-α-fluoro group and a 16-α-methyl
substituent).
A variety of synthetic glucocorticoids, some far more potent than
cortisol, have been created for therapeutic use. They differ in both pharmacokinetics (absorption factor, half-life, volume of distribution, clearance) and pharmacodynamics (for example the capacity of mineralocorticoid activity: retention of sodium (Na+) and water; renal physiology). Because they permeate the intestines easily, they are administered primarily per os (by mouth), but also by other methods, such as topically on skin. More than 90% of them bind different plasma proteins,
though with a different binding specificity. Endogenous glucocorticoids
and some synthetic corticoids have high affinity to the protein transcortin (also called corticosteroid-binding globulin), whereas all of them bind albumin. In the liver, they quickly metabolize by conjugation with a sulfate or glucuronic acid, and are secreted in the urine.
Glucocorticoid potency, duration of effect, and the overlapping mineralocorticoid potency vary. Cortisol is the standard of comparison for glucocorticoid potency. Hydrocortisone is the name used for pharmaceutical preparations of cortisol.
The data below refer to oral administration. Oral potency may be less than parenteral potency because significant amounts (up to 50% in some cases) may not reach the circulation. Fludrocortisone acetate and deoxycorticosterone acetate
are, by definition, mineralocorticoids rather than glucocorticoids, but
they do have minor glucocorticoid potency and are included in this
table to provide perspective on mineralocorticoid potency.
| Name | Glucocorticoid potency |
Mineralocorticoid potency |
Terminal half-life (hours) |
|---|---|---|---|
| Cortisol (hydrocortisone) | 1 | 1 | 8 |
| Cortisone | 0.8 | 0.8 | 8 |
| Prednisone | 3.5–5 | 0.8 | 16–36 |
| Prednisolone | 4 | 0.8 | 16–36 |
| Methylprednisolone | 5–7.5 | 0.5 | 18–40 |
| Dexamethasone | 25–80 | 0 | 36–54 |
| Betamethasone | 25–30 | 0 | 36–54 |
| Triamcinolone | 5 | 0 | 12–36 |
| Fludrocortisone acetate | 15 | 200 | 24 |
| Deoxycorticosterone acetate | 0 | 20 | - |
Therapeutic use
Glucocorticoids may be used in low doses in adrenal insufficiency. In much higher doses, oral or inhaled glucocorticoids are used to suppress various allergic, inflammatory, and autoimmune disorders. Inhaled glucocorticoids are the second-line treatment for asthma. They are also administered as post-transplantory immunosuppressants to prevent the acute transplant rejection and the graft-versus-host disease. Nevertheless, they do not prevent an infection and also inhibit later reparative processes. Newly emerging evidence showed that glucocorticoids could be used in the treatment of heart failure
to increase the renal responsiveness to diuretics and natriuretic
peptides. Glucocorticoids are historically used for pain relief in inflammatory conditions. However, corticosteroids show limited efficacy in pain relief and potential adverse events for their use in tendinopathies.
Physiological replacement
Any
glucocorticoid can be given in a dose that provides approximately the
same glucocorticoid effects as normal cortisol production; this is
referred to as physiologic, replacement, or maintenance dosing. This is
approximately 6–12 mg/m2/day of hydrocortisone (m2 refers to body surface area (BSA), and is a measure of body size; an average man's BSA is 1.9 m2).
Therapeutic immunosuppression
Glucocorticoids cause immunosuppression, and the therapeutic component of this effect is mainly the decreases in the function and numbers of lymphocytes, including both B cells and T cells.
The major mechanism for this immunosuppression is through
inhibition of nuclear factor kappa-light-chain-enhancer of activated B
cells (NF-κB).
NF-κB is a critical transcription factor involved in the synthesis of
many mediators (i.e., cytokines) and proteins (i.e., adhesion proteins)
that promote the immune response. Inhibition of this transcription
factor, therefore, blunts the capacity of the immune system to mount a
response.
Glucocorticoids suppress cell-mediated immunity by inhibiting genes that code for the cytokines IL-1, IL-2, IL-3, IL-4, IL-5, IL-6, IL-8 and IFN-γ, the most important of which is IL-2. Smaller cytokine production reduces the T cell proliferation.
Glucocorticoids, however, not only reduce T cell proliferation,
but also lead to another well known effect - glucocorticoid-induced
apoptosis. The effect is more prominent in immature T cells still inside
in the thymus, but peripheral T cells are also affected. The exact
mechanism regulating this glucocorticoid sensitivity lies in the Bcl-2 gene.
Glucocorticoids also suppress the humoral immunity, thereby causing a humoral immune deficiency. Glucocorticoids cause B cells to express smaller amounts of IL-2 and of IL-2 receptors. This diminishes both B cell clone expansion and antibody synthesis. The diminished amounts of IL-2 also cause fewer T lymphocyte cells to be activated.
The effect of glucocorticoids on Fc receptor expression in immune cells is complicated. Dexamethasone decreases IFN-gamma stimulated Fc gamma RI expression in neutrophils while conversely causing an increase in monocytes. Glucocorticoids may also decrease the expression of Fc receptors in macrophages, but the evidence supporting this regulation in earlier studies has been questioned. The effect of Fc receptor expression in macrophages is important since it is necessary for the phagocytosis of opsonised cells. This is because Fc receptors bind antibodies attached to cells targeted for destruction by macrophages.
Anti-inflammatory
Glucocorticoids
are potent anti-inflammatories, regardless of the inflammation's cause;
their primary anti-inflammatory mechanism is lipocortin-1 (annexin-1) synthesis. Lipocortin-1 both suppresses phospholipase A2, thereby blocking eicosanoid production, and inhibits various leukocyte inflammatory events (epithelial adhesion, emigration, chemotaxis, phagocytosis, respiratory burst,
etc.). In other words, glucocorticoids not only suppress immune
response, but also inhibit the two main products of inflammation, prostaglandins and leukotrienes. They inhibit prostaglandin synthesis at the level of phospholipase A2 as well as at the level of cyclooxygenase/PGE isomerase (COX-1 and COX-2), the latter effect being much like that of NSAIDs, thus potentiating the anti-inflammatory effect.
In addition, glucocorticoids also suppress cyclooxygenase expression.
Glucocorticoids marketed as anti-inflammatories are often topical formulations, such as nasal sprays for rhinitis or inhalers for asthma.
These preparations have the advantage of only affecting the targeted
area, thereby reducing side effects or potential interactions. In this
case, the main compounds used are beclometasone, budesonide, fluticasone, mometasone and ciclesonide. In rhinitis, sprays are used. For asthma, glucocorticoids are administered as inhalants with a metered-dose or dry powder inhaler.
Hyperaldosteronism
Glucocorticoids can be used in the management of familial hyperaldosteronism type 1. They are not effective, however, for use in the type 2 condition.
Resistance
Corticosteroid resistance mechanisms
Resistance to the therapeutic uses of glucocorticoids can present difficulty; for instance, 25% of cases of severe asthma
may be unresponsive to steroids. This may be the result of genetic
predisposition, ongoing exposure to the cause of the inflammation (such
as allergens),
immunological phenomena that bypass glucocorticoids, and
pharmacokinetic disturbances (incomplete absorption or accelerated
excretion or metabolism).
Heart failure
Glucocorticoids
could be used in the treatment of decompensated heart failure to
potentiate renal responsiveness to diuretics, especially in heart
failure patients with refractory diuretic resistance with large doses of
loop diuretics.
Side effects
Glucocorticoid
drugs currently being used act nonselectively, so in the long run they
may impair many healthy anabolic processes. To prevent this, much
research has been focused recently on the elaboration of selectively
acting glucocorticoid drugs. Side effects include:
- Immunodeficiency (see section below)
- Hyperglycemia due to increased gluconeogenesis, insulin resistance, and impaired glucose tolerance ("steroid diabetes"); caution in those with diabetes mellitus
- Increased skin fragility, easy bruising
- Negative calcium balance due to reduced intestinal calcium absorption
- Steroid-induced osteoporosis: reduced bone density (osteoporosis, osteonecrosis, higher fracture risk, slower fracture repair)
- Weight gain due to increased visceral and truncal fat deposition (central obesity) and appetite stimulation
- Hypercortisolemia with prolonged or excessive use (also known as, exogenous Cushing's syndrome)
- Impaired memory and attention deficits
- Adrenal insufficiency (if used for long time and stopped suddenly without a taper)
- Muscle and tendon breakdown (proteolysis), weakness, reduced muscle mass and repair
- Expansion of malar fat pads and dilation of small blood vessels in skin
- Lipomatosis within the epidural space
- Excitatory effect on central nervous system (euphoria, psychosis)
- Anovulation, irregularity of menstrual periods
- Growth failure, delayed puberty
- Increased plasma amino acids, increased urea formation, negative nitrogen balance
- Glaucoma due to increased ocular pressure
- Cataracts
- Topical steroid addiction
In high doses, hydrocortisone (cortisol) and those glucocorticoids
with appreciable mineralocorticoid potency can exert a mineralocorticoid
effect as well, although in physiologic doses this is prevented by
rapid degradation of cortisol by 11β-hydroxysteroid dehydrogenase isoenzyme 2 (11β-HSD2) in mineralocorticoid target tissues. Mineralocorticoid effects can include salt and water retention, extracellular fluid volume expansion, hypertension, potassium depletion, and metabolic alkalosis.
Immunodeficiency
Glucocorticoids cause immunosuppression, decreasing the function and/or numbers of neutrophils, lymphocytes (including both B cells and T cells), monocytes, macrophages, and the anatomical barrier function of the skin. This suppression, if large enough, can cause manifestations of immunodeficiency, including T cell deficiency, humoral immune deficiency and neutropenia.
| Bacteria | |
|---|---|
| Fungi | |
| Viruses | |
| Other |
Withdrawal
In addition to the effects listed above, use of high-dose steroids
for more than a week begins to produce suppression of the patient's adrenal glands because the exogenous glucocorticoids suppress hypothalamic corticotropin-releasing hormone and pituitary adrenocorticotropic hormone.
With prolonged suppression, the adrenal glands atrophy (physically
shrink), and can take months to recover full function after
discontinuation of the exogenous glucocorticoid.
During this recovery time, the patient is vulnerable to adrenal insufficiency
during times of stress, such as illness. While suppressive dose and
time for adrenal recovery vary widely, clinical guidelines have been
devised to estimate potential adrenal suppression and recovery, to
reduce risk to the patient. The following is one example:
- If patients have been receiving daily high doses for five days or less, they can be abruptly stopped (or reduced to physiologic replacement if patients are adrenal-deficient). Full adrenal recovery can be assumed to occur by a week afterward.
- If high doses were used for six to 10 days, reduce to replacement dose immediately and taper over four more days. Adrenal recovery can be assumed to occur within two to four weeks of completion of steroids.
- If high doses were used for 11–30 days, cut immediately to twice replacement, and then by 25% every four days. Stop entirely when dose is less than half of replacement. Full adrenal recovery should occur within one to three months of completion of withdrawal.
- If high doses were used more than 30 days, cut dose immediately to twice replacement, and reduce by 25% each week until replacement is reached. Then change to oral hydrocortisone or cortisone as a single morning dose, and gradually decrease by 2.5 mg each week. When the morning dose is less than replacement, the return of normal basal adrenal function may be documented by checking 0800 cortisol levels prior to the morning dose; stop drugs when 0800 cortisol is 10 μg/dl. Predicting the time to full adrenal recovery after prolonged suppressive exogenous steroids is difficult; some people may take nearly a year.
- Flare-up of the underlying condition for which steroids are given may require a more gradual taper than outlined above.