From Wikipedia, the free encyclopedia

The Baltoro Glacier in the Karakoram, Baltistan, Northern Pakistan. At 62 kilometres (39 mi) in length, it is one of the longest alpine glaciers on earth.
A glacier (US /ˈɡleɪʃər/ or UK /ˈɡlæsiə/) is a persistent body of dense ice that is constantly moving under its own weight; it forms where the accumulation of snow exceeds its ablation (melting and sublimation) over many years, often centuries. Glaciers slowly deform and flow due to stresses induced by their weight, creating crevasses, seracs, and other distinguishing features. They also abrade rock and debris from their substrate to create landforms such as cirques and moraines. Glaciers form only on land and are distinct from the much thinner sea ice and lake ice that form on the surface of bodies of water.
On Earth, 99% of glacial ice is contained within vast ice sheets in the polar regions, but glaciers may be found in mountain ranges on every continent except Australia, and on a few high-latitude oceanic islands. Between 35°N and 35°S, glaciers occur only in the Himalayas, Andes, Rocky Mountains, a few high mountains in East Africa, Mexico, New Guinea and on Zard Kuh in Iran.[1]
Glacial ice is the largest reservoir of freshwater on Earth.[2] Many glaciers from temperate, alpine and seasonal polar climates store water as ice during the colder seasons and release it later in the form of meltwater as warmer summer temperatures cause the glacier to melt, creating a water source that is especially important for plants, animals and human uses when other sources may be scant. Within high altitude and Antarctic environments, the seasonal temperature difference is often not sufficient to release meltwater.
Because glacial mass is affected by long-term climate changes, e.g., precipitation, mean temperature, and cloud cover, glacial mass changes are considered among the most sensitive indicators of climate change and are a major source of variations in sea level.
The word glacier comes from French. It is derived from the Vulgar Latin glacia and ultimately from Latin glacies meaning "ice".[3] The processes and features caused by glaciers and related to them are referred to as glacial. The process of glacier establishment, growth and flow is called glaciation. The corresponding area of study is called glaciology. Glaciers are important components of the global cryosphere.
Types
Glaciers are categorized by their morphology, thermal characteristics, and behavior. Alpine glaciers, also known as mountain glaciers or cirque glaciers, form on the crests and slopes of mountains. An alpine glacier that fills a valley is sometimes called a valley glacier. A large body of glacial ice astride a mountain, mountain range, or volcano is termed an ice cap or ice field.[4] Ice caps have an area less than 50,000 km² (20,000 mile²) by definition.
Glacial bodies larger than 50,000 km² are called ice sheets or continental glaciers.[5] Several kilometers deep, they obscure the underlying topography. Only nunataks protrude from their surfaces. The only extant ice sheets are the two that cover most of Antarctica and Greenland. They contain vast quantities of fresh water, enough that if both melted, global sea levels would rise by over 70 meters.[6] Portions of an ice sheet or cap that extend into water are called ice shelves; they tend to be thin with limited slopes and reduced velocities.[7] Narrow, fast-moving sections of an ice sheet are called ice streams.[8][9] In Antarctica, many ice streams drain into large ice shelves. Some drain directly into the sea, often with an ice tongue, like Mertz Glacier.
Tidewater glaciers are glaciers that terminate in the sea, including most glaciers flowing from Greenland, Antarctica, Baffin and Ellesmere Islands in Canada, Southeast Alaska, and the Northern and Southern Patagonian Ice Fields. As the ice reaches the sea, pieces break off, or calve, forming icebergs. Most tidewater glaciers calve above sea level, which often results in a tremendous impact as the iceberg strikes the water. Tidewater glaciers undergo centuries-long cycles of advance and retreat that are much less affected by the climate change than those of other glaciers.
Thermally, a temperate glacier is at melting point throughout the year, from its surface to its base. The ice of a polar glacier is always below freezing point from the surface to its base, although the surface snowpack may experience seasonal melting. A sub-polar glacier includes both temperate and polar ice, depending on depth beneath the surface and position along the length of the glacier. In a similar way, the thermal regime of a glacier is often described by the temperature at its base alone. A cold-based glacier is below freezing at the ice-ground interface, and is thus frozen to the underlying substrate. A warm-based glacier is above or at freezing at the interface, and is able to slide at this contact.[10] This contrast is thought to a large extent to govern the ability of a glacier to effectively erode its bed, as sliding ice promotes plucking at rock from the surface below.[11] Glaciers which are partly cold-based and partly warm-based are known as polythermal.[10]
Formation
Glaciers form where the accumulation of snow and ice exceeds ablation. The area in which a glacier forms is called a cirque (corrie or cwm) - a typically armchair-shaped geological feature (such as a depression between mountains enclosed by arêtes) - which collects and compresses through gravity the snow which falls into it. This snow collects and is compacted by the weight of the snow falling above it forming névé. Further crushing of the individual snowflakes and squeezing the air from the snow turns it into 'glacial ice'. This glacial ice will fill the cirque until it 'overflows' through a geological weakness or vacancy, such as the gap between two mountains. When the mass of snow and ice is sufficiently thick, it begins to move due to a combination of surface slope, gravity and pressure. On steeper slopes, this can occur with as little as 15 m (50 ft) of snow-ice.
In temperate glaciers, snow repeatedly freezes and thaws, changing into granular ice called firn. Under the pressure of the layers of ice and snow above it, this granular ice fuses into denser and denser firn. Over a period of years, layers of firn undergo further compaction and become glacial ice. Glacier ice is slightly less dense than ice formed from frozen water because it contains tiny trapped air bubbles.
Glacial ice has a distinctive blue tint because it absorbs some red light due to an overtone of the infrared OH stretching mode of the water molecule. Liquid water is blue for the same reason. The blue of glacier ice is sometimes misattributed to Rayleigh scattering due to bubbles in the ice.[12]
Structure
A glacier originates at a location called its glacier head and terminates at its glacier foot, snout, or terminus.Glaciers are broken into zones based on surface snowpack and melt conditions.[13] The ablation zone is the region where there is a net loss in glacier mass. The equilibrium line separates the ablation zone and the accumulation zone; it is the altitude where the amount of new snow gained by accumulation is equal to the amount of ice lost through ablation. The upper part of a glacier, where accumulation exceeds ablation, is called the accumulation zone. In general, the accumulation zone accounts for 60–70% of the glacier's surface area, more if the glacier calves icebergs. Ice in the accumulation zone is deep enough to exert a downward force that erodes underlying rock. After a glacier melts, it often leaves behind a bowl- or amphitheater-shaped depression that ranges in size from large basins like the Great Lakes to smaller mountain depressions known as cirques.
The accumulation zone can be subdivided based on its melt conditions.
- The dry snow zone is a region where no melt occurs, even in the summer, and the snowpack remains dry.
- The percolation zone is an area with some surface melt, causing meltwater to percolate into the snowpack. This zone is often marked by refrozen ice lenses, glands, and layers. The snowpack also never reaches melting point.
- Near the equilibrium line on some glaciers, a superimposed ice zone develops. This zone is where meltwater refreezes as a cold layer in the glacier, forming a continuous mass of ice.
- The wet snow zone is the region where all of the snow deposited since the end of the previous summer has been raised to 0 °C.
Following the Little Ice Age's end around 1850, glaciers around the Earth have retreated substantially. A slight cooling led to the advance of many alpine glaciers between 1950–1985, but since 1985 glacier retreat and mass loss has become larger and increasingly ubiquitous.[14][15][16]
Motion

Shear or herring-bone crevasses on Emmons Glacier (Mount Rainier); such crevasses often form near the edge of a glacier where interactions with underlying or marginal rock impede flow. In this case, the impediment appears to be some distance from the near margin of the glacier.
Glaciers also move through basal sliding. In this process, a glacier slides over the terrain on which it sits, lubricated by the presence of liquid water. The water is created from ice that melts under high pressure from frictional heating. Basal sliding is dominant in temperate, or warm-based glaciers.
Fracture zone and cracks
The top 50 metres (160 ft) of a glacier are rigid because they are under low pressure. This upper section is known as the fracture zone; it mostly moves as a single unit over the plastically flowing lower section. When a glacier moves through irregular terrain, cracks called crevasses develop in the fracture zone. Crevasses form due to differences in glacier velocity. If two rigid sections of a glacier move at different speeds and directions, shear forces cause them to break apart, opening a crevasse. Crevasses are seldom more than 150 feet (46 m) deep but in some cases can be 1,000 feet (300 m) or even deeper. Beneath this point, the plasticity of the ice is too great for cracks to form. Intersecting crevasses can create isolated peaks in the ice, called seracs.
Crevasses can form in several different ways. Transverse crevasses are transverse to flow and form where steeper slopes cause a glacier to accelerate. Longitudinal crevasses form semi-parallel to flow where a glacier expands laterally. Marginal crevasses form from the edge of the glacier, due to the reduction in speed caused by friction of the valley walls. Marginal crevasses are usually largely transverse to flow. Moving glacier ice can sometimes separate from stagnant ice above, forming a bergschrund. Bergschrunds resemble crevasses but are singular features at a glacier's margins.
Crevasses make travel over glaciers hazardous, especially when they are hidden by fragile snow bridges.
Below the equilibrium line, glacial meltwater is concentrated in stream channels. Meltwater can pool in proglacial lakes on top of a glacier or descend into the depths of a glacier via moulins. Streams within or beneath a glacier flow in englacial or sub-glacial tunnels. These tunnels sometimes reemerge at the glacier's surface.[19]
Speed
The speed of glacial displacement is partly determined by friction. Friction makes the ice at the bottom of the glacier move more slowly than ice at the top. In alpine glaciers, friction is also generated at the valley's side walls, which slows the edges relative to the center.Mean speeds vary greatly, but is typically around 1 meter per day.[20] There may be no motion in stagnant areas; for example, in parts of Alaska, trees can establish themselves on surface sediment deposits. In other cases, glaciers can move as fast as 20–30 m per day, such as in Greenland's Jakobshavn Isbræ (Greenlandic: Sermeq Kujalleq). Velocity increases with increasing slope, increasing thickness, increasing snowfall, increasing longitudinal confinement, increasing basal temperature, increasing meltwater production and reduced bed hardness.
A few glaciers have periods of very rapid advancement called surges. These glaciers exhibit normal movement until suddenly they accelerate, then return to their previous state. During these surges, the glacier may reach velocities far greater than normal speed.[21] These surges may be caused by failure of the underlying bedrock, the pooling of meltwater at the base of the glacier[22] — perhaps delivered from a supraglacial lake — or the simple accumulation of mass beyond a critical "tipping point".[23]
In glaciated areas where the glacier moves faster than one km per year, glacial earthquakes occur. These are large scale temblors that have seismic magnitudes as high as 6.1.[24][25] The number of glacial earthquakes in Greenland peaks every year in July, August and September and is increasing over time. In a study using data from January 1993 through October 2005, more events were detected every year since 2002, and twice as many events were recorded in 2005 as there were in any other year. This increase in the numbers of glacial earthquakes in Greenland may be a response to global warming.[24][25]
Ogives
Ogives are alternating wave crests and valleys that appear as dark and light bands of ice on glacier surfaces. They are linked to seasonal motion of glaciers; the width of one dark and one light band generally equals the annual movement of the glacier. Ogives are formed when ice from an icefall is severely broken up, increasing ablation surface area during summer. This creates a swale and space for snow accumulation in the winter, which in turn creates a ridge.[26] Sometimes ogives consist only of undulations or color bands and are described as wave ogives or band ogives.[27]Geography

Black ice glacier near Aconcagua, Argentina
Glaciers are present on every continent and approximately fifty countries, excluding those (Australia, South Africa) that have glaciers only on distant subantarctic island territories. Extensive glaciers are found in Antarctica, Chile, Canada, Alaska, Greenland and Iceland. Mountain glaciers are widespread, especially in the Andes, the Himalayas, the Rocky Mountains, the Caucasus, and the Alps. Mainland Australia currently contains no glaciers, although a small glacier on Mount Kosciuszko was present in the last glacial period.[28] In New Guinea, small, rapidly diminishing, glaciers are located on its highest summit massif of Puncak Jaya.[29] Africa has glaciers on Mount Kilimanjaro in Tanzania, on Mount Kenya and in the Rwenzori Mountains. Oceanic islands with glaciers occur on Iceland, Svalbard, New Zealand, Jan Mayen and the subantarctic islands of Marion, Heard, Grande Terre (Kerguelen) and Bouvet. During glacial periods of the Quaternary, Taiwan, Hawaii on Mauna Kea[30] and Tenerife also had large alpine glaciers, while the Faroe and Crozet Islands[31] were completely glaciated.
The permanent snow cover necessary for glacier formation is affected by factors such as the degree of slope on the land, amount of snowfall and the winds. Glaciers can be found in all latitudes except from 20° to 27° north and south of the equator where the presence of the descending limb of the Hadley circulation lowers precipitation so much that with high insolation snow lines reach above 6,500 metres (21,330 ft). Between 19˚N and 19˚S, however, precipitation is higher and the mountains above 5,000 metres (16,400 ft) usually have permanent snow.
Even at high latitudes, glacier formation is not inevitable. Areas of the Arctic, such as Banks Island, and the McMurdo Dry Valleys in Antarctica are considered polar deserts where glaciers cannot form because they receive little snowfall despite the bitter cold. Cold air, unlike warm air, is unable to transport much water vapor. Even during glacial periods of the Quaternary, Manchuria, lowland Siberia,[32] and central and northern Alaska,[33] though extraordinarily cold, had such light snowfall that glaciers could not form.[34][35]
In addition to the dry, unglaciated polar regions, some mountains and volcanoes in Bolivia, Chile and Argentina are high (4,500 metres (14,800 ft) - 6,900 m (22,600 ft)) and cold, but the relative lack of precipitation prevents snow from accumulating into glaciers. This is because these peaks are located near or in the hyperarid Atacama Desert.
Glacial geology
Glaciers erode terrain through two principal processes: abrasion and plucking.
As glaciers flow over bedrock, they soften and lift blocks of rock into the ice. This process, called plucking, is caused by subglacial water that penetrates fractures in the bedrock and subsequently freezes and expands. This expansion causes the ice to act as a lever that loosens the rock by lifting it. Thus, sediments of all sizes become part of the glacier's load. If a retreating glacier gains enough debris, it may become a rock glacier, like the Timpanogos Glacier in Utah.
Abrasion occurs when the ice and its load of rock fragments slide over bedrock and function as sandpaper, smoothing and polishing the bedrock below. The pulverized rock this process produces is called rock flour and is made up of rock grains between 0.002 and 0.00625 mm in size. Abrasion leads to steeper valley walls and mountain slopes in alpine settings, which can cause avalanches and rock slides. These add even more material to the glacier.
Glacial abrasion is commonly characterized by glacial striations. Glaciers produce these when they contain large boulders that carve long scratches in the bedrock. By mapping the direction of the striations, researchers can determine the direction of the glacier's movement. Similar to striations are chatter marks, lines of crescent-shape depressions in the rock underlying a glacier. They are formed by abrasion when boulders in the glacier are repeatedly caught and released as they are dragged along the bedrock.
The rate of glacier erosion is variable. Six factors control erosion rate:
- Velocity of glacial movement
- Thickness of the ice
- Shape, abundance and hardness of rock fragments contained in the ice at the bottom of the glacier
- Relative ease of erosion of the surface under the glacier
- Thermal conditions at the glacier base
- Permeability and water pressure at the glacier base
- Glacial till: material directly deposited from glacial ice. Till includes a mixture of undifferentiated material ranging from clay size to boulders, the usual composition of a moraine.
- Fluvial and outwash sediments: sediments deposited by water. These deposits are stratified by size.
Moraines
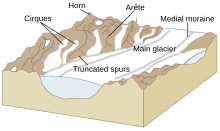
Glacial moraines are formed by the deposition of material from a glacier and are exposed after the glacier has retreated. They usually appear as linear mounds of till, a non-sorted mixture of rock, gravel and boulders within a matrix of a fine powdery material. Terminal or end moraines are formed at the foot or terminal end of a glacier. Lateral moraines are formed on the sides of the glacier. Medial moraines are formed when two different glaciers merge and the lateral moraines of each coalesce to form a moraine in the middle of the combined glacier. Less apparent are ground moraines, also called glacial drift, which often blankets the surface underneath the glacier downslope from the equilibrium line.
The term moraine is of French origin. It was coined by peasants to describe alluvial embankments and rims found near the margins of glaciers in the French Alps. In modern geology, the term is used more broadly, and is applied to a series of formations, all of which are composed of till. Moraines can also create moraine dammed lakes.
Drumlins
Drumlins are asymmetrical, canoe shaped hills made mainly of till. Their heights vary from 15 to 50 meters and they can reach a kilometer in length. The steepest side of the hill faces the direction from which the ice advanced (stoss), while a longer slope is left in the ice's direction of movement (lee).
Drumlins are found in groups called drumlin fields or drumlin camps. One of these fields is found east of Rochester, New York; it is estimated to contain about 10,000 drumlins.
Although the process that forms drumlins is not fully understood, their shape implies that they are products of the plastic deformation zone of ancient glaciers. It is believed that many drumlins were formed when glaciers advanced over and altered the deposits of earlier glaciers.
Glacial valleys, cirques, arêtes, and pyramidal peaks
Before glaciation, mountain valleys have a characteristic "V" shape, produced by eroding water. During glaciation, these valleys are widened, deepened, and smoothed, forming a "U"-shaped glacial valley. The erosion that creates glacial valleys eliminates the spurs of earth that extend across mountain valleys, creating triangular cliffs called truncated spurs. Within glacial valleys, depressions created by plucking and abrasion can be filled by lakes, called paternoster lakes. If a glacial valley runs into a large body of water, it forms a fjord.
Many glaciers deepen their valleys more than their smaller tributaries. Therefore, when glaciers recede, the valleys of the tributary glaciers remain above the main glacier's depression and are called hanging valleys.
At the start of a classic valley glacier is a bowl-shaped cirque, which has escarped walls on three sides but is open on the side that descends into the valley. Cirques are where ice begins to accumulate in a glacier. Two glacial cirques may form back to back and erode their backwalls until only a narrow ridge, called an arête is left. This structure may result in a mountain pass. If multiple cirques encircle a single mountain, they create pointed pyramidal peaks; particularly steep examples are called horns.
Roche moutonnée
Some rock formations in the path of a glacier are sculpted into small hills called roche moutonnée, or "sheepback" rock. Roche moutonnée are elongated, rounded, and asymmetrical bedrock knobs can be produced by glacier erosion. They range in length from less than a meter to several hundred meters long.[36] Roche moutonnée have a gentle slope on their up-glacier sides and a steep to vertical face on their down-glacier sides. The glacier abrades the smooth slope on the upstream side as it flows along, but tears loose and carries away rock from the downstream side via plucking.Alluvial stratification
As the water that rises from the ablation zone moves away from the glacier, it carries fine eroded sediments with it. As the speed of the water decreases, so does its capacity to carry objects in suspension. The water thus gradually deposits the sediment as it runs, creating an alluvial plain. When this phenomenon occurs in a valley, it is called a valley train. When the deposition is in an estuary, the sediments are known as bay mud.Outwash plains and valley trains are usually accompanied by basins known as "kettles". These are small lakes formed when large ice blocks that are trapped in alluvium melt and produce water-filled depressions. Kettle diameters range from 5 m to 13 km, with depths of up to 45 meters. Most are circular in shape because the blocks of ice that formed them were rounded as they melted.[37]
Glacial deposits
When a glacier's size shrinks below a critical point, its flow stops and it becomes stationary. Meanwhile, meltwater within and beneath the ice leaves stratified alluvial deposits. These deposits, in the forms of columns, terraces and clusters, remain after the glacier melts and are known as "glacial deposits".
Glacial deposits that take the shape of hills or mounds are called kames. Some kames form when meltwater deposits sediments through openings in the interior of the ice. Others are produced by fans or deltas created by meltwater. When the glacial ice occupies a valley, it can form terraces or kames along the sides of the valley.
Long, sinuous glacial deposits are called eskers. Eskers are composed of sand and gravel that was deposited by meltwater streams that flowed through ice tunnels within or beneath a glacier. They remain after the ice melts, with heights exceeding 100 meters and lengths of as long as 100 km.
Loess deposits
Very fine glacial sediments or rock flour is often picked up by wind blowing over the bare surface and may be deposited great distances from the original fluvial deposition site. These eolian loess deposits may be very deep, even hundreds of meters, as in areas of China and the Midwestern United States of America. Katabatic winds can be important in this process.Isostatic rebound

Isostatic pressure by a glacier on the Earth's crust
Large masses, such as ice sheets or glaciers, can depress the crust of the Earth into the mantle. The depression usually totals a third of the ice sheet or glacier's thickness. After the ice sheet or glacier melts, the mantle begins to flow back to its original position, pushing the crust back up. This post-glacial rebound, which proceeds very slowly after the melting of the ice sheet or glacier, is currently occurring in measurable amounts in Scandinavia and the Great Lakes region of North America.
A geomorphological feature created by the same process on a smaller scale is known as dilation-faulting. It occurs where previously compressed rock is allowed to return to its original shape more rapidly than can be maintained without faulting. This leads to an effect similar to what would be seen if the rock were hit by a large hammer. Dilation faulting can be observed in recently de-glaciated parts of Iceland and Cumbria.
On Mars
At mid-latitudes, between 35° and 65° north or south, Martian glaciers are affected by the thin Martian atmosphere. Because of the low atmospheric pressure, ablation near the surface is solely due to sublimation, not melting. As on Earth, many glaciers are covered with a layer of rocks which insulates the ice. A radar instrument on board the Mars Reconnaissance Orbiter found ice under a thin layer of rocks in formations called lobate debris aprons (LDAs).[40][41][42][43][44]
The pictures below illustrate how landscape features on Mars closely resemble those on the Earth.
-
Mesa in Ismenius Lacus quadrangle, as seen by CTX. Mesa has several glaciers eroding it. One of the glaciers is seen in greater detail in the next two images from HiRISE. Image from Ismenius Lacus quadrangle.
-
Glacier as seen by HiRISE under the HiWish program. Area in rectangle is enlarged in the next photo. Zone of accumulation of snow at the top. Glacier is moving down valley, then spreading out on plain. Evidence for flow comes from the many lines on surface. Location is in Protonilus Mensae in Ismenius Lacus quadrangle.
-
Enlargement of area in rectangle of the previous image. On Earth the ridge would be called the terminal moraine of an alpine glacier. Picture taken with HiRISE under the HiWish program. Image from Ismenius Lacus quadrangle.