| Adeno-associated virus | |
|---|---|
| |
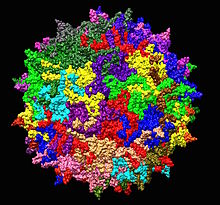
| Adeno-associated virus serotype 2 structure from 1LP3. One fivefold axis shown center. | |
| Scientific classification | |
| (unranked): | Virus |
| Realm: | Monodnaviria |
| Kingdom: | Shotokuvirae |
| Phylum: | Cossaviricota |
| Class: | Quintoviricetes |
| Order: | Piccovirales |
| Family: | Parvoviridae |
| Subfamily: | Parvovirinae |
| Genus: | Dependoparvovirus |
| Viruses included: | |
| |
Adeno-associated viruses (AAV) are small viruses that infect humans and some other primate species. They belong to the genus Dependoparvovirus, which in turn belongs to the family Parvoviridae. They are small (20 nm) replication-defective, nonenveloped viruses.
AAV are not currently known to cause disease. The viruses cause a very mild immune response. Several additional features make AAV an attractive candidate for creating viral vectors for gene therapy, and for the creation of isogenic human disease models. Gene therapy vectors using AAV can infect both dividing and quiescent cells and persist in an extrachromosomal state without integrating into the genome of the host cell, although in the native virus integration of virally carried genes into the host genome does occur.
Integration can be important for certain applications, but can also
have unwanted consequences. Recent human clinical trials using AAV for gene therapy in the retina have shown promise.
History
The adeno-associated virus (AAV), previously thought to be a contaminant in adenovirus preparations, was first identified as a dependoparvovirus in the 1960s in the laboratories of Bob Atchison at Pittsburgh and Wallace Rowe at NIH. Serological studies in humans subsequently indicated that, despite being present in people infected by helper viruses such as adenovirus or herpes virus, AAV itself did not cause any disease.
Use in gene therapy
Advantages and drawbacks
Wild-type
AAV has attracted considerable interest from gene therapy researchers
due to a number of features. Chief amongst these is the virus's apparent
lack of pathogenicity. It can also infect non-dividing cells and has
the ability to stably integrate into the host cell genome at a specific
site (designated AAVS1) in the human chromosome 19. This feature makes it somewhat more predictable than retroviruses, which present the threat of a random insertion and of mutagenesis, which is sometimes followed by development of a cancer.
The AAV genome integrates most frequently into the site mentioned,
while random incorporations into the genome take place with a negligible
frequency. Development of AAVs as gene therapy vectors, however, has
eliminated this integrative capacity by removal of the rep and cap from the DNA of the vector. The desired gene together with a promoter to drive transcription of the gene is inserted between the inverted terminal repeats (ITRs) that aid in concatemer
formation in the nucleus after the single-stranded vector DNA is
converted by host cell DNA polymerase complexes into double-stranded
DNA. AAV-based gene therapy vectors form episomal
concatemers in the host cell nucleus. In non-dividing cells, these
concatemers remain intact for the life of the host cell. In dividing
cells, AAV DNA is lost through cell division, since the episomal DNA is
not replicated along with the host cell DNA. Random integration of AAV DNA into the host genome is detectable but occurs at very low frequency. AAVs also present very low immunogenicity, seemingly restricted to generation of neutralizing antibodies, while they induce no clearly defined cytotoxic response. This feature, along with the ability to infect quiescent cells present their dominance over adenoviruses as vectors for human gene therapy.
Use of the virus does present some disadvantages. The cloning
capacity of the vector is relatively limited and most therapeutic genes
require the complete replacement of the virus's 4.8 kilobase genome.
Large genes are, therefore, not suitable for use in a standard AAV
vector. Options are currently being explored to overcome the limited
coding capacity.
The AAV ITRs of two genomes can anneal to form head-to-tail
concatemers, almost doubling the capacity of the vector. Insertion of
splice sites allows for the removal of the ITRs from the transcript.
Because of AAV's specialized gene therapy advantages, researchers have created an altered version of AAV termed self-complementary adeno-associated virus (scAAV).
Whereas AAV packages a single strand of DNA and must wait for its
second strand to be synthesized, scAAV packages two shorter strands that
are complementary to each other. By avoiding second-strand synthesis,
scAAV can express more quickly, although as a caveat, scAAV can only
encode half of the already limited capacity of AAV.
Recent reports suggest that scAAV vectors are more immunogenic than
single stranded adenovirus vectors, inducing a stronger activation of cytotoxic T lymphocytes.
Humoral immunity instigated by infection with the wild type is
thought to be common. The associated neutralising activity limits the
usefulness of the most commonly used serotype AAV2 in certain
applications. Accordingly, the majority of clinical trials under way
involve delivery of AAV2 into the brain, a relatively immunologically
privileged organ. In the brain, AAV2 is strongly neuron-specific.
Clinical trials
To
date, AAV vectors have been used in over 117 clinical trials worldwide,
approximately 5.6% of virus-vectored gene-therapy trials. Recently, promising results have been obtained from Phase 1 and Phase 2 trials for a number of diseases, including Leber's congenital amaurosis, hemophilia, congestive heart failure, spinal muscular atrophy, lipoprotein lipase deficiency, and Parkinson's disease.
| Indication | Gene | Route of administration | Phase | Subject number | Status |
| Cystic fibrosis | CFTR | Lung, via aerosol | I | 12 | Complete |
| CFTR | Lung, via aerosol | II | 38 | Complete | |
| CFTR | Lung, via aerosol | II | 100 | Complete | |
| Hemophilia B | FIX | Intramuscular | I | 9 | Complete |
| FIX | Hepatic artery | I | 6 | Ended | |
| Arthritis | TNFR:Fc | Intraarticular | I | 1 | Ongoing |
| Hereditary emphysema | AAT | Intramuscular | I | 12 | Ongoing |
| Leber's congenital amaurosis | RPE65 | Subretinal | I–II | Multiple | Several ongoing and complete |
| Age-related macular degeneration | sFlt-1 | Subretinal | I–II | 24 | Ongoing |
| Duchenne muscular dystrophy | SGCA | Intramuscular | I | 10 | Ongoing |
| Parkinson's disease | GAD65, GAD67 | Intracranial | I | 12 | Complete |
| Canavan disease | AAC | Intracranial | I | 21 | Ongoing |
| Batten disease | CLN2 | Intracranial | I | 10 | Ongoing |
| Alzheimer's disease | NGF | Intracranial | I | 6 | Ongoing |
| Spinal muscular atrophy | SMN1 | Intravenous and Intrathecal | I–III | 15 | Several ongoing and complete |
| Congestive heart failure | SERCA2a | Intra-coronary | IIb | 250 | Ongoing |
Structure
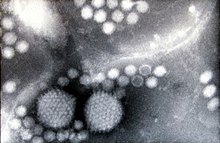
Two
adenovirus particles surrounded by numerous, smaller adeno-associated
viruses (negative-staining electron microscopy, magnification
approximately 200,000×)
Genome, transcriptome and proteome
The AAV genome is built of single-stranded deoxyribonucleic acid (ssDNA),
either positive- or negative-sensed, which is about 4.7 kilobase long.
The genome comprises ITRs at both ends of the DNA strand, and two open reading frames (ORFs): rep and cap. The former is composed of four overlapping genes encoding Rep proteins required for the AAV life cycle, and the latter contains overlapping nucleotide sequences of capsid proteins: VP1, VP2 and VP3, which interact to form a capsid with icosahedral symmetry.
ITR sequences
The
inverted terminal repeat (ITR) sequences comprise 145 bases each. They
were named so because of their symmetry, which was shown to be required
for efficient multiplication of the AAV genome. The feature of these sequences that gives them this property is their ability to form a hairpin, which contributes to so-called self-priming that allows primase-independent
synthesis of the second DNA strand. The ITRs were also shown to be
required for both integration of the AAV DNA into the host cell genome
(19th chromosome in humans) and rescue from it, as well as for efficient encapsidation of the AAV DNA combined with generation of a fully assembled, deoxyribonuclease-resistant AAV particles.
With regard to gene therapy, ITRs seem to be the only sequences required in cis next to the therapeutic gene: structural (cap) and packaging (rep) proteins can be delivered in trans. With this assumption many methods were established for efficient production of recombinant AAV (rAAV) vectors containing a reporter or therapeutic gene. However, it was also published that the ITRs are not the only elements required in cis for the effective replication and encapsidation. A few research groups have identified a sequence designated cis-acting Rep-dependent element (CARE) inside the coding sequence of the rep gene. CARE was shown to augment the replication and encapsidation when present in cis.
rep gene and Rep proteins
On the "left side" of the genome there are two promoters called p5 and p19, from which two overlapping messenger ribonucleic acids (mRNAs) of different length can be produced. Each of these contains an intron which can be either spliced
out or not. Given these possibilities, four various mRNAs, and
consequently four various Rep proteins with overlapping sequence can be
synthesized. Their names depict their sizes in kilodaltons (kDa): Rep78, Rep68, Rep52 and Rep40. Rep78 and 68 can specifically bind the hairpin
formed by the ITR in the self-priming act and cleave at a specific
region, designated terminal resolution site, within the hairpin. They
were also shown to be necessary for the AAVS1-specific integration of
the AAV genome. All four Rep proteins were shown to bind ATP and to possess helicase
activity. It was also shown that they upregulate the transcription from
the p40 promoter (mentioned below), but downregulate both p5 and p19
promoters.
cap gene and VP proteins
The
right side of a positive-sensed AAV genome encodes overlapping
sequences of three capsid proteins, VP1, VP2 and VP3, which start from
one promoter, designated p40. The molecular weights of these proteins
are 87, 72 and 62 kiloDaltons, respectively. The AAV capsid is composed of a mixture of VP1, VP2, and VP3 totaling 60 monomers arranged in icosahedral symmetry in a ratio of 1:1:10, with an estimated size of 3.9 MegaDaltons.
The crystal structure of the VP3 protein was determined by Xie, Bue, et al.
AAV2 capsid, shown as a ribbon diagram, with the back half hidden for clarity. One fivefold symmetry axis is shown center.
The cap gene produces an additional, non-structural protein
called the Assembly-Activating Protein (AAP). This protein is produced
from ORF2 and is essential for the capsid-assembly process. The exact function of this protein in the assembly process and its structure have not been solved to date.
All three VPs are translated from one mRNA. After this mRNA is synthesized, it can be spliced in two different manners: either a longer or shorter intron
can be excised resulting in the formation of two pools of mRNAs: a 2.3
kb- and a 2.6 kb-long mRNA pool. Usually, especially in the presence of adenovirus, the longer intron is preferred, so the 2.3-kb-long mRNA represents the so-called "major splice". In this form the first AUG codon,
from which the synthesis of VP1 protein starts, is cut out, resulting
in a reduced overall level of VP1 protein synthesis. The first AUG codon
that remains in the major splice is the initiation codon for VP3
protein. However, upstream of that codon in the same open reading frame
lies an ACG sequence (encoding threonine) which is surrounded by an
optimal Kozak context.
This contributes to a low level of synthesis of VP2 protein, which is
actually VP3 protein with additional N terminal residues, as is VP1.
Since the bigger intron is preferred to be spliced out, and since in the major splice the ACG codon is a much weaker translation initiation signal, the ratio at which the AAV structural proteins are synthesized in vivo is about 1:1:20, which is the same as in the mature virus particle. The unique fragment at the N terminus of VP1 protein was shown to possess the phospholipase A2 (PLA2) activity, which is probably required for the releasing of AAV particles from late endosomes. Muralidhar et al. reported that VP2 and VP3 are crucial for correct virion assembly. More recently, however, Warrington et al.
showed VP2 to be unnecessary for the complete virus particle formation
and an efficient infectivity, and also presented that VP2 can tolerate
large insertions in its N terminus, while VP1 can not, probably because
of the PLA2 domain presence.
Classification, serotypes, receptors and native tropism
Two species of AAV were recognised by the International Committee on Taxonomy of Viruses in 2013: adeno-associated dependoparvovirus A (formerly AAV-1, -2, -3 and -4) and adeno-associated dependoparvovirus B (formerly AAV-5).
Until the 1990s, virtually all AAV biology was studied using AAV
serotype 2. However, AAV is highly prevalent in humans and other
primates and several serotypes have been isolated from various tissue
samples. Serotypes 2, 3, 5, and 6 were discovered in human cells, AAV
serotypes 1, 4, and 7–11 in nonhuman primate samples. As of 2006 there have been 11 AAV serotypes described, the 11th in 2004.
AAV capsid proteins contain 12 hypervariable surface regions, with most
variability occurring in the threefold proximal peaks, but the
parvovirus genome in general presents highly conserved replication and
structural genes across serotypes.
All of the known serotypes can infect cells from multiple diverse
tissue types. Tissue specificity is determined by the capsid serotype
and pseudotyping of AAV vectors to alter their tropism range will likely
be important to their use in therapy.
Serotype 2
Serotype 2 (AAV2) has been the most extensively examined so far. AAV2 presents natural tropism towards skeletal muscles, neurons, vascular smooth muscle cells and hepatocytes.
Three cell receptors have been described for AAV2: heparan sulfate proteoglycan (HSPG), aVβ5 integrin and fibroblast growth factor
receptor 1 (FGFR-1). The first functions as a primary receptor, while
the latter two have a co-receptor activity and enable AAV to enter the
cell by receptor-mediated endocytosis. These study results have been disputed by Qiu, Handa, et al. HSPG functions as the primary receptor, though its abundance in the extracellular matrix can scavenge AAV particles and impair the infection efficiency.
Studies have shown that serotype 2 of the virus (AAV-2)
apparently kills cancer cells without harming healthy ones. "Our
results suggest that adeno-associated virus type 2, which infects the
majority of the population but has no known ill effects, kills multiple
types of cancer cells yet has no effect on healthy cells," said Craig
Meyers, a professor of immunology and microbiology at the Penn State College of Medicine in Pennsylvania in 2005. This could lead to a new anti-cancer agent.
Other serotypes
Although
AAV2 is the most popular serotype in various AAV-based research, it has
been shown that other serotypes can be more effective as gene delivery
vectors. For instance AAV6 appears much better in infecting airway
epithelial cells,
AAV7 presents very high transduction rate of murine skeletal muscle
cells (similar to AAV1 and AAV5), AAV8 is superb in transducing
hepatocytes and AAV1 and 5 were shown to be very efficient in gene delivery to vascular endothelial cells. In the brain, most AAV serotypes show neuronal tropism, while AAV5 also transduces astrocytes. AAV6, a hybrid of AAV1 and AAV2, also shows lower immunogenicity than AAV2.
Serotypes can differ with the respect to the receptors they are
bound to. For example, AAV4 and AAV5 transduction can be inhibited by
soluble sialic acids (of different form for each of these serotypes), and AAV5 was shown to enter cells via the platelet-derived growth factor receptor.
Synthetic serotypes
There
have been many efforts to engineer and improve new AAV variants for
both clinical and research purposes. Such modifications include new
tropisms to target specific tissues, and modified surface residues to
evade detection by the immune system. Beyond opting for particular
strains of recombinant AAV
(rAAV) to target particular cells, researchers have also explored AAV
pseudotyping, the practice of creating hybrids of certain AAV strains to
approach an even more refined target. The hybrid is created by taking a
capsid from one strain and the genome from another strain. For example,
research involving AAV2/5, a hybrid with the genome of AAV2 and the
capsid of AAV5, was able to achieve more accuracy and range in brain
cells than AAV2 would be able to achieve unhybridized. Researchers have
continued to experiment with pseudotyping by creating strains with
hybrid capsids. AAV-DJ has a hybrid capsid from eight different strains
of AAV; as such, it can infect different cells throughout many areas of
the body, a property which a single strain of AAV with a limited tropism
would not have.
Other efforts to engineer and improve new AAV variants have involved
the ancestral reconstruction of virus variants to generate new vectors
with enhanced properties for clinical applications and the study of AAV
biology.
Immunology
AAV
is of particular interest to gene therapists due to its apparent
limited capacity to induce immune responses in humans, a factor which
should positively influence vector transduction efficiency while
reducing the risk of any immune-associated pathology.
AAV is not considered to have any known role in disease.
Innate
The innate
immune response to the AAV vectors has been characterised in animal
models. Intravenous administration in mice causes transient production
of pro-inflammatory cytokines and some infiltration of neutrophils and other leukocytes
into the liver, which seems to sequester a large percentage of the
injected viral particles. Both soluble factor levels and cell
infiltration appear to return to baseline within six hours. By contrast,
more aggressive viruses produce innate responses lasting 24 hours or
longer.
Humoral
The virus is known to instigate robust humoral immunity in animal models and in the human population, where up to 80% of individuals are thought to be seropositive for AAV2. Antibodies
are known to be neutralising, and for gene therapy applications these
do impact on vector transduction efficiency via some routes of
administration. As well as persistent AAV specific antibody levels, it
appears from both prime-boost studies in animals and from clinical
trials that the B-cell memory is also strong. In seropositive humans, circulating IgG antibodies for AAV2 appear to be primarily composed of the IgG1 and IgG2 subclasses, with little or no IgG3 or IgG4 present.
Cell-mediated
The cell-mediated
response to the virus and to vectors is poorly characterised, and has
been largely ignored in the literature as recently as 2005.
Clinical trials using an AAV2-based vector to treat haemophilia B seem
to indicate that targeted destruction of transduced cells may be
occurring. Combined with data that shows that CD8+ T-cells can recognise elements of the AAV capsid in vitro,
it appears that there may be a cytotoxic T lymphocyte response to AAV
vectors. Cytotoxic responses would imply the involvement of CD4+ T helper cells in the response to AAV and in vitro data from human studies suggests that the virus may indeed induce such responses, including both Th1 and Th2 memory responses. A number of candidate T cell stimulating epitopes
have been identified within the AAV capsid protein VP1, which may be
attractive targets for modification of the capsid if the virus is to be
used as a vector for gene therapy.
Infection cycle
There are several steps in the AAV infection cycle, from infecting a cell to producing new infectious particles:
- attachment to the cell membrane
- receptor-mediated endocytosis
- endosomal trafficking
- escape from the late endosome or lysosome
- translocation to the nucleus
- uncoating
- formation of double-stranded DNA replicative form of the AAV genome
- expression of rep genes
- genome replication
- expression of cap genes, synthesis of progeny ssDNA particles
- assembly of complete virions, and
- release from the infected cell.
Some of these steps may look different in various types of cells,
which, in part, contributes to the defined and quite limited native
tropism of AAV. Replication of the virus can also vary in one cell type,
depending on the cell's current cell cycle phase.
The characteristic feature of the adeno-associated virus is a
deficiency in replication and thus its inability to multiply in
unaffected cells. Adeno-associated virus spreads by co-infecting a cell
with a helper virus. The first helper virus that was described as
providing successful generation of new AAV particles, was the adenovirus,
from which the AAV name originated. It was then shown that AAV
replication can be facilitated by selected proteins derived from the
adenovirus genome, by other viruses such as HSV or vaccinia, or by genotoxic agents, such as UV irradiation or hydroxyurea.
Depending on the presence or absence of a helper virus, the life cycle
of AAV follows either a lytic or lysogenic pathway, respectively.
If there is a helper virus, AAV's gene expression activates, allowing
the virus to replicate using the host cell's polymerase. When the helper
virus kills the host cell, the new AAV virions are released. If there
is not a helper virus present, AAV exhibits lysogenic behavior. When AAV
infects a cell alone, its gene expression is repressed (AAV does not
replicate), and its genome is incorporated into the host genome (into
human chromosome 19). In rare cases, lysis can occur without a helper
virus, but usually AAV can not replicate and kill a cell on its own.
The minimal set of the adenoviral genes required for efficient
generation, of progeny AAV particles, was discovered by Matsushita,
Ellinger et al.
This discovery allowed for new production methods of recombinant AAV,
which do not require adenoviral co-infection of the AAV-producing cells.
In the absence of helper virus or genotoxic factors, AAV DNA can
either integrate into the host genome or persist in episomal
form. In the former case integration is mediated by Rep78 and Rep68
proteins and requires the presence of ITRs flanking the region being
integrated. In mice, the AAV genome has been observed persisting for
long periods of time in quiescent tissues, such as skeletal muscles, in
episomal form (a circular head-to-tail conformation).