In thermodynamics, the Joule–Thomson effect (also known as the Joule–Kelvin effect, Kelvin–Joule effect) describes the temperature change of a real gas or liquid (as differentiated from an ideal gas) when it is forced through a valve or porous plug while keeping them insulated so that no heat is exchanged with the environment. This procedure is called a throttling process or Joule–Thomson process. At room temperature, all gases except hydrogen, helium and neon
cool upon expansion by the Joule–Thomson process when being throttled
through an orifice; these three gases experience the same effect but
only at lower temperatures. Some liquids such as hydraulic oils will be warmed by the Joule-Thomson throttling process.
The gas-cooling throttling process is commonly exploited in refrigeration processes such as air conditioners, heat pumps, and liquefiers. In hydraulics, the warming effect from Joule-Thomson throttling can be used to find internally leaking valves as these will produce heat which can be detected by thermocouple or thermal-imaging camera. Throttling is a fundamentally irreversible process. The throttling due to the flow resistance in supply lines, heat exchangers, regenerators, and other components of (thermal) machines is a source of losses that limits the performance.
The gas-cooling throttling process is commonly exploited in refrigeration processes such as air conditioners, heat pumps, and liquefiers. In hydraulics, the warming effect from Joule-Thomson throttling can be used to find internally leaking valves as these will produce heat which can be detected by thermocouple or thermal-imaging camera. Throttling is a fundamentally irreversible process. The throttling due to the flow resistance in supply lines, heat exchangers, regenerators, and other components of (thermal) machines is a source of losses that limits the performance.
History
Description
The adiabatic (no heat exchanged) expansion of a gas may be carried out in a number of ways. The change in temperature experienced by the gas during expansion depends not only on the initial and final pressure, but also on the manner in which the expansion is carried out.- If the expansion process is reversible, meaning that the gas is in thermodynamic equilibrium at all times, it is called an isentropic expansion. In this scenario, the gas does positive work during the expansion, and its temperature decreases.
- In a free expansion, on the other hand, the gas does no work and absorbs no heat, so the internal energy is conserved. Expanded in this manner, the temperature of an ideal gas would remain constant, but the temperature of a real gas decreases, except at very high temperature.
- The method of expansion discussed in this article, in which a gas or liquid at pressure P1 flows into a region of lower pressure P2 without significant change in kinetic energy, is called the Joule–Thomson expansion. The expansion is inherently irreversible. During this expansion, enthalpy remains unchanged. Unlike a free expansion, work is done, causing a change in internal energy. Whether the internal energy increases or decreases is determined by whether work is done on or by the fluid; that is determined by the initial and final states of the expansion and the properties of the fluid.
Sign of the Joule–Thomson coefficient, for N2. Within the region bounded by the red line, a Joule–Thomson expansion produces cooling ();
outside that region, the expansion produces heating. The gas–liquid
coexistence curve is shown by the blue line, terminating at the critical
point (the solid blue circle). The dashed lines demarcates the regions
where N2 is a supercritical fluid, a liquid, or a gas.
The temperature change produced during a Joule–Thomson expansion is quantified by the Joule–Thomson coefficient, . This coefficient may be either positive (corresponding to cooling) or negative (heating); the regions where each occurs for molecular nitrogen, N2, are shown in the figure. Note that most conditions in the figure correspond to N2 being a supercritical fluid, where it has some properties of a gas and some of a liquid, but can not be really described as being either. The coefficient is negative at both very high and very low temperatures; at very high pressure it is negative at all temperatures. The maximum inversion temperature (621 K for N2) occurs as zero pressure is approached. For N2 gas at low pressures, is negative at high temperatures and positive at low temperatures. At temperatures below the gas-liquid coexistence curve, N2 condenses to form a liquid and the coefficient again becomes negative. Thus, for N2 gas below 621 K, a Joule–Thomson expansion can be used to cool the gas until liquid N2 forms.
Physical mechanism
There are two factors that can change the temperature of a fluid during an adiabatic expansion: a change in internal energy or the conversion between potential and kinetic internal energy. Temperature is the measure of thermal kinetic energy (energy associated with molecular motion); so a change in temperature indicates a change in thermal kinetic energy. The internal energy is the sum of thermal kinetic energy and thermal potential energy. Thus, even if the internal energy does not change, the temperature can change due to conversion between kinetic and potential energy; this is what happens in a free expansion and typically produces a decrease in temperature as the fluid expands. If work is done on or by the fluid as it expands, then the total internal energy changes. This is what happens in a Joule–Thomson expansion and can produce larger heating or cooling than observed in a free expansion.In a Joule–Thomson expansion the enthalpy remains constant. The enthalpy, , is defined as
For an ideal gas, does not change during a Joule–Thomson expansion. As a result, there is no change in internal energy; since there is also no change in thermal potential energy, there can be no change in thermal kinetic energy and, therefore, no change in temperature. In real gases, does change.
The ratio of the value of to that expected for an ideal gas at the same temperature is called the compressibility factor, . For a gas, this is typically less than unity at low temperature and greater than unity at high temperature. At low pressure, the value of always moves towards unity as a gas expands. Thus at low temperature, and will increase as the gas expands, resulting in a positive Joule–Thomson coefficient. At high temperature, and decrease as the gas expands; if the decrease is large enough, the Joule–Thomson coefficient will be negative.
For liquids, and for supercritical fluids under high pressure, increases as pressure increases. This is due to molecules being forced together, so that the volume can barely decrease due to higher pressure. Under such conditions, the Joule–Thomson coefficient is negative, as seen in the figure above.
The physical mechanism associated with the Joule–Thomson effect is closely related to that of a shock wave, although a shock wave differs in that the change in bulk kinetic energy of the gas flow is not negligible.
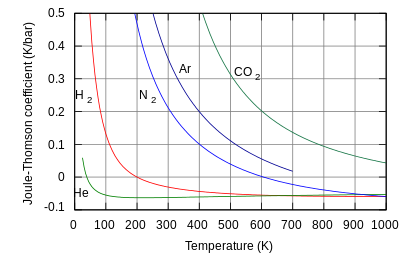
The Joule–Thomson (Kelvin) coefficient
Fig. 1 – Joule–Thomson coefficients for various gases at atmospheric pressure.
The rate of change of temperature with respect to pressure in a Joule–Thomson process (that is, at constant enthalpy ) is the Joule–Thomson (Kelvin) coefficient . This coefficient can be expressed in terms of the gas's volume , its heat capacity at constant pressure , and its coefficient of thermal expansion as:
All real gases have an inversion point at which the value of changes sign. The temperature of this point, the Joule–Thomson inversion temperature, depends on the pressure of the gas before expansion.
In a gas expansion the pressure decreases, so the sign of is negative by definition. With that in mind, the following table explains when the Joule–Thomson effect cools or warms a real gas:
| If the gas temperature is | then is | since is | thus must be | so the gas |
|---|---|---|---|---|
| below the inversion temperature | positive | always negative | negative | cools |
| above the inversion temperature | negative | always negative | positive | warms |
Helium and hydrogen are two gases whose Joule–Thomson inversion temperatures at a pressure of one atmosphere are very low (e.g., about 45 K (−228 °C) for helium). Thus, helium and hydrogen warm when expanded at constant enthalpy at typical room temperatures. On the other hand, nitrogen and oxygen, the two most abundant gases in air, have inversion temperatures of 621 K (348 °C) and 764 K (491 °C) respectively: these gases can be cooled from room temperature by the Joule–Thomson effect.
For an ideal gas, is always equal to zero: ideal gases neither warm nor cool upon being expanded at constant enthalpy.
Applications
In practice, the Joule–Thomson effect is achieved by allowing the gas to expand through a throttling device (usually a valve) which must be very well insulated to prevent any heat transfer to or from the gas. No external work is extracted from the gas during the expansion (the gas must not be expanded through a turbine, for example).The cooling produced in the Joule–Thomson expansion makes it a valuable tool in refrigeration. The effect is applied in the Linde technique as a standard process in the petrochemical industry, where the cooling effect is used to liquefy gases, and also in many cryogenic applications (e.g. for the production of liquid oxygen, nitrogen, and argon). A gas must be below its inversion temperature to be liquefied by the Linde cycle. For this reason, simple Linde cycle liquefiers, starting from ambient temperature, cannot be used to liquefy helium, hydrogen, or neon. However, the Joule–Thomson effect can be used to liquefy even helium, provided that the helium gas is first cooled below its inversion temperature of 40 K.
Proof that the specific enthalpy remains constant
In thermodynamics so-called "specific" quantities are quantities per unit mass (kg) and are denoted by lower-case characters. So h, u, and v are the specific enthalpy, specific internal energy, and specific volume (volume per unit mass, or reciprocal density), respectively. In a Joule–Thomson process the specific enthalpy h remains constant. To prove this, the first step is to compute the net work done when a mass m of the gas moves through the plug. This amount of gas has a volume of V1 = m v1 in the region at pressure P1 (region 1) and a volume V2 = m v2 when in the region at pressure P2 (region 2). Then in region 1, the "flow work" done on the amount of gas by the rest of the gas is: W1 = m P1v1. In region 2, the work done by the amount of gas on the rest of the gas is: W2 = m P2v2. So, the total work done on the mass m of gas isThrottling in the T-s diagram
Fig. 2 – T-s
diagram of nitrogen. The red dome represents the
two-phase region with
the low-entropy side (the saturated liquid)
and the high-entropy side
(the saturated gas). The black curves
give the T-s
relation along isobars. The pressures are indicated in
bar. The blue
curves are isenthalps (curves of constant specific enthalpy). The
specific enthalpies are indicated in kJ/kg. The
specific points a, b,
etc., are treated in the main text.
A very convenient way to get a quantitative understanding of the throttling process is by using diagrams. There are many types of diagrams (h-T diagram, h-P diagram, etc.) Commonly used are the so-called T-s diagrams. Figure 2 shows the T-s diagram of nitrogen as an example. Various points are indicated as follows:
- a) T = 300 K, p = 200 bar, s = 5.16 kJ/(kgK), h = 430 kJ/kg;
- b) T = 270 K, p = 1 bar, s = 6.79 kJ/(kgK), h = 430 kJ/kg;
- c) T = 133 K, p = 200 bar, s = 3.75 kJ/(kgK), h = 150 kJ/kg;
- d) T = 77.2 K, p = 1 bar, s = 4.40 kJ/(kgK), h = 150 kJ/kg;
- e) T = 77.2 K, p = 1 bar, s = 2.83 kJ/(kgK), h = 28 kJ/kg (saturated liquid at 1 bar);
- f) T = 77.2 K, p = 1 bar, s = 5.41 kJ/(kgK), h =230 kJ/kg (saturated gas at 1 bar).
Derivation of the Joule–Thomson coefficient
It is difficult to think physically about what the Joule–Thomson coefficient, , represents. Also, modern determinations of do not use the original method used by Joule and Thomson, but instead measure a different, closely related quantity. Thus, it is useful to derive relationships between and other, more convenient quantities. That is the purpose of this section.The first step in obtaining these results is to note that the Joule–Thomson coefficient involves the three variables T, P, and H. A useful result is immediately obtained by applying the cyclic rule; in terms of these three variables that rule may be written
- .
To proceed further, the starting point is the fundamental equation of thermodynamics in terms of enthalpy; this is
Joule's second law
It is easy to verify that for an ideal gas defined by suitable microscopic postulates that αT = 1, so the temperature change of such an ideal gas at a Joule–Thomson expansion is zero. For such an ideal gas, this theoretical result implies that:- The internal energy of a fixed mass of an ideal gas depends only on its temperature (not pressure or volume).