An overview of electromagnetic radiation absorption. This example discusses the general principle using visible light as a specific example. A white beam source – emitting light of multiple wavelengths – is focused on a sample (the complementary color pairs are indicated by the yellow dotted lines). Upon striking the sample, photons that match the energy gap of the molecules present (green light in this example) are absorbed
in order to excite the molecule. Other photons transmit unaffected
and, if the radiation is in the visible region (400-700nm), the sample
color is the complementary color of the absorbed light. By comparing
the attenuation of the transmitted light with the incident, an absorption spectrum can be obtained.
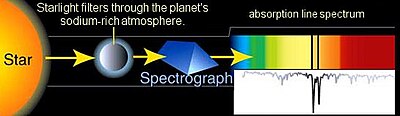
The first direct detection and chemical analysis of the atmosphere of an exoplanet, in 2001. Sodium in the atmosphere filters the starlight of HD 209458 as the giant planet passes in front of the star.
Absorption spectroscopy refers to spectroscopic techniques that measure the absorption of radiation, as a function of frequency or wavelength,
due to its interaction with a sample. The sample absorbs energy, i.e.,
photons, from the radiating field. The intensity of the absorption
varies as a function of frequency, and this variation is the absorption spectrum. Absorption spectroscopy is performed across the electromagnetic spectrum.
Absorption spectroscopy is employed as an analytical chemistry
tool to determine the presence of a particular substance in a sample
and, in many cases, to quantify the amount of the substance present. Infrared and ultraviolet-visible spectroscopy
are particularly common in analytical applications. Absorption
spectroscopy is also employed in studies of molecular and atomic
physics, astronomical spectroscopy and remote sensing.
There are a wide range of experimental approaches for measuring
absorption spectra. The most common arrangement is to direct a generated
beam of radiation at a sample and detect the intensity of the radiation
that passes through it. The transmitted energy can be used to calculate
the absorption. The source, sample arrangement and detection technique
vary significantly depending on the frequency range and the purpose of
the experiment.
Absorption spectrum
Solar spectrum with Fraunhofer lines as it appears visually.
A material's absorption spectrum is the fraction of incident
radiation absorbed by the material over a range of frequencies. The
absorption spectrum is primarily determined by the atomic and molecular
composition of the material. Radiation is more likely to be absorbed at
frequencies that match the energy difference between two quantum mechanical states of the molecules. The absorption that occurs due to a transition between two states is referred to as an absorption line and a spectrum is typically composed of many lines.
The frequencies where absorption lines occur, as well as their relative intensities, primarily depend on the electronic and molecular structure of the sample. The frequencies will also depend on the interactions between molecules in the sample, the crystal structure in solids, and on several environmental factors (e.g., temperature, pressure, electromagnetic field). The lines will also have a width and shape that are primarily determined by the spectral density or the density of states of the system.
Theory
Absorption lines are typically classified by the nature of the quantum mechanical change induced in the molecule or atom. Rotational lines,
for instance, occur when the rotational state of a molecule is changed.
Rotational lines are typically found in the microwave spectral region. Vibrational lines
correspond to changes in the vibrational state of the molecule and are
typically found in the infrared region. Electronic lines correspond to a
change in the electronic state of an atom or molecule and are typically
found in the visible and ultraviolet region. X-ray absorption is
associated with the excitation of inner shell electrons in atoms. These changes can also be combined (e.g. rotation-vibration transitions), leading to new absorption lines at the combined energy of the two changes.
The energy associated with the quantum mechanical change
primarily determines the frequency of the absorption line but the
frequency can be shifted by several types of interactions. Electric and
magnetic fields can cause a shift. Interactions with neighboring
molecules can cause shifts. For instance, absorption lines of the gas
phase molecule can shift significantly when that molecule is in a liquid
or solid phase and interacting more strongly with neighboring
molecules.
The width and shape of absorption lines are determined by the
instrument used for the observation, the material absorbing the
radiation and the physical environment of that material. It is common
for lines to have the shape of a Gaussian or Lorentzian distribution. It is also common for a line to be described solely by its intensity and width instead of the entire shape being characterized.
The integrated intensity—obtained by integrating
the area under the absorption line—is proportional to the amount of the
absorbing substance present. The intensity is also related to the
temperature of the substance and the quantum mechanical interaction
between the radiation and the absorber. This interaction is quantified
by the transition moment and depends on the particular lower state the transition starts from, and the upper state it is connected to.
The width of absorption lines may be determined by the spectrometer used to record it. A spectrometer has an inherent limit on how narrow a line it can resolve
and so the observed width may be at this limit. If the width is larger
than the resolution limit, then it is primarily determined by the
environment of the absorber. A liquid or solid absorber, in which
neighboring molecules strongly interact with one another, tends to have
broader absorption lines than a gas. Increasing the temperature or
pressure of the absorbing material will also tend to increase the line
width. It is also common for several neighboring transitions to be close
enough to one another that their lines overlap and the resulting
overall line is therefore broader yet.
Relation to transmission spectrum
Absorption
and transmission spectra represent equivalent information and one can
be calculated from the other through a mathematical transformation. A
transmission spectrum will have its maximum intensities at wavelengths
where the absorption is weakest because more light is transmitted
through the sample. An absorption spectrum will have its maximum
intensities at wavelengths where the absorption is strongest.
Relation to emission spectrum
Emission spectrum of iron
Emission
is a process by which a substance releases energy in the form of
electromagnetic radiation. Emission can occur at any frequency at which
absorption can occur, and this allows the absorption lines to be
determined from an emission spectrum. The emission spectrum
will typically have a quite different intensity pattern from the
absorption spectrum, though, so the two are not equivalent. The
absorption spectrum can be calculated from the emission spectrum using
appropriate theoretical models and additional information about the
quantum mechanical states of the substance.
Relation to scattering and reflection spectra
The scattering and reflection spectra of a material are influenced by both its index of refraction and its absorption spectrum. In an optical context, the absorption spectrum is typically quantified by the extinction coefficient, and the extinction and index coefficients are quantitatively related through the Kramers-Kronig relation.
Therefore, the absorption spectrum can be derived from a scattering or
reflection spectrum. This typically requires simplifying assumptions or
models, and so the derived absorption spectrum is an approximation.
Applications
The infrared absorption spectrum of NASA laboratory sulfur dioxide ice is compared with the infrared absorption spectra of ices on Jupiter's moon, Io credit NASA, Bernard Schmitt, and UKIRT.
Absorption spectroscopy is useful in chemical analysis
because of its specificity and its quantitative nature. The specificity
of absorption spectra allows compounds to be distinguished from one
another in a mixture, making absorption spectroscopy useful in wide
variety of applications. For instance, Infrared gas analyzers
can be used to identify the presence of pollutants in the air,
distinguishing the pollutant from nitrogen, oxygen, water and other
expected constituents.
The specificity also allows unknown samples to be identified by
comparing a measured spectrum with a library of reference spectra. In
many cases, it is possible to determine qualitative information about a
sample even if it is not in a library. Infrared spectra, for instance,
have characteristics absorption bands that indicate if carbon-hydrogen
or carbon-oxygen bonds are present.
An absorption spectrum can be quantitatively related to the amount of material present using the Beer-Lambert law. Determining the absolute concentration of a compound requires knowledge of the compound's absorption coefficient.
The absorption coefficient for some compounds is available from
reference sources, and it can also be determined by measuring the
spectrum of a calibration standard with a known concentration of the
target.
Remote sensing
One
of the unique advantages of spectroscopy as an analytical technique is
that measurements can be made without bringing the instrument and sample
into contact. Radiation that travels between a sample and an instrument
will contain the spectral information, so the measurement can be made remotely.
Remote spectral sensing is valuable in many situations. For example,
measurements can be made in toxic or hazardous environments without
placing an operator or instrument at risk. Also, sample material does
not have to be brought into contact with the instrument—preventing
possible cross contamination.
Remote spectral measurements present several challenges compared
to laboratory measurements. The space in between the sample of interest
and the instrument may also have spectral absorptions. These absorptions
can mask or confound the absorption spectrum of the sample. These
background interferences may also vary over time. The source of
radiation in remote measurements is often an environmental source, such
as sunlight or the thermal radiation from a warm object, and this makes
it necessary to distinguish spectral absorption from changes in the
source spectrum.
To simplify these challenges, Differential optical absorption spectroscopy
has gained some popularity, as it focuses on differential absorption
features and omits broad-band absorption such as aerosol extinction and
extinction due to Rayleigh scattering. This method is applied to
ground-based, air-borne and satellite based measurements. Some
ground-based methods provide the possibility to retrieve tropospheric
and stratospheric trace gas profiles.
Astronomy
Absorption spectrum observed by the Hubble Space Telescope
Astronomical spectroscopy
is a particularly significant type of remote spectral sensing. In this
case, the objects and samples of interest are so distant from earth that
electromagnetic radiation is the only means available to measure them.
Astronomical spectra contain both absorption and emission spectral
information. Absorption spectroscopy has been particularly important for
understanding interstellar clouds and determining that some of them contain molecules. Absorption spectroscopy is also employed in the study of extrasolar planets. Detection of extrasolar planets by the transit method also measures their absorption spectrum and allows for the determination of the planet's atmospheric composition, temperature, pressure, and scale height, and hence allows also for the determination of the planet's mass.
Atomic and molecular physics
Theoretical models, principally quantum mechanical models, allow for the absorption spectra of atoms and molecules to be related to other physical properties such as electronic structure, atomic or molecular mass, and molecular geometry. Therefore, measurements of the absorption spectrum are used to determine these other properties. Microwave spectroscopy, for example, allows for the determination of bond lengths and angles with high precision.
In addition, spectral measurements can be used to determine the accuracy of theoretical predictions. For example, the Lamb shift measured in the hydrogen
atomic absorption spectrum was not expected to exist at the time it was
measured. Its discovery spurred and guided the development of quantum electrodynamics, and measurements of the Lamb shift are now used to determine the fine-structure constant.
Experimental methods
Basic approach
The
most straightforward approach to absorption spectroscopy is to generate
radiation with a source, measure a reference spectrum of that radiation
with a detector
and then re-measure the sample spectrum after placing the material of
interest in between the source and detector. The two measured spectra
can then be combined to determine the material's absorption spectrum.
The sample spectrum alone is not sufficient to determine the absorption
spectrum because it will be affected by the experimental conditions—the
spectrum of the source, the absorption spectra of other materials in
between the source and detector and the wavelength dependent
characteristics of the detector. The reference spectrum will be affected
in the same way, though, by these experimental conditions and therefore
the combination yields the absorption spectrum of the material alone.
A wide variety of radiation sources are employed in order to
cover the electromagnetic spectrum. For spectroscopy, it is generally
desirable for a source to cover a broad swath of wavelengths in order to
measure a broad region of the absorption spectrum. Some sources
inherently emit a broad spectrum. Examples of these include globars or other black body sources in the infrared, mercury lamps in the visible and ultraviolet and x-ray tubes. One recently developed, novel source of broad spectrum radiation is synchrotron radiation
which covers all of these spectral regions. Other radiation sources
generate a narrow spectrum but the emission wavelength can be tuned to
cover a spectral range. Examples of these include klystrons in the microwave region and lasers across the infrared, visible and ultraviolet region (though not all lasers have tunable wavelengths).
The detector employed to measure the radiation power will also
depend on the wavelength range of interest. Most detectors are sensitive
to a fairly broad spectral range and the sensor selected will often
depend more on the sensitivity and noise requirements of a given
measurement. Examples of detectors common in spectroscopy include heterodyne receivers in the microwave, bolometers in the millimeter-wave and infrared, mercury cadmium telluride and other cooled semiconductor detectors in the infrared, and photodiodes and photomultiplier tubes in the visible and ultraviolet.
If both the source and the detector cover a broad spectral region, then it is also necessary to introduce a means of resolving the wavelength of the radiation in order to determine the spectrum. Often a spectrograph
is used to spatially separate the wavelengths of radiation so that the
power at each wavelength can be measured independently. It is also
common to employ interferometry to determine the spectrum—Fourier transform infrared spectroscopy is a widely used implementation of this technique.
Two other issues that must be considered in setting up an absorption spectroscopy experiment include the optics used to direct the radiation and the means of holding or containing the sample material (called a cuvette
or cell). For most UV, visible, and NIR measurements the use of
precision quartz cuvettes are necessary. In both cases, it is important
to select materials that have relatively little absorption of their own
in the wavelength range of interest. The absorption of other materials
could interfere with or mask the absorption from the sample. For
instance, in several wavelength ranges it is necessary to measure the
sample under vacuum or in a rare gas environment because gases in the atmosphere have interfering absorption features.
Specific approaches
- Astronomical spectroscopy
- Cavity ring down spectroscopy (CRDS)
- Laser absorption spectrometry (LAS)
- Mössbauer spectroscopy
- Photoemission spectroscopy
- Photothermal optical microscopy
- Photothermal spectroscopy
- Reflectance spectroscopy
- Tunable diode laser absorption spectroscopy (TDLAS)
- X-ray absorption fine structure (XAFS)
- X-ray absorption near edge structure (XANES)
- Total absorption spectroscopy (TAS)