OVIRS instrument of the Osiris-REx probe is a visible and infrared spectrometer
Infrared spectroscopy (IR spectroscopy or vibrational spectroscopy) involves the interaction of infrared radiation with matter. It covers a range of techniques, mostly based on absorption spectroscopy. As with all spectroscopic techniques, it can be used to identify and study chemicals.
Samples may be solid, liquid, or gas. The method or technique of
infrared spectroscopy is conducted with an instrument called an infrared spectrometer (or spectrophotometer) to produce an infrared spectrum. An IR spectrum can be visualized in a graph of infrared light absorbance (or transmittance) on the vertical axis vs. frequency or wavelength on the horizontal axis. Typical units of frequency used in IR spectra are reciprocal centimeters (sometimes called wave numbers), with the symbol cm−1. Units of IR wavelength are commonly given in micrometers (formerly called "microns"), symbol μm, which are related to wave numbers in a reciprocal way. A common laboratory instrument that uses this technique is a Fourier transform infrared (FTIR) spectrometer. Two-dimensional IR is also possible as discussed below.
The infrared portion of the electromagnetic spectrum is usually divided into three regions; the near-,
mid- and far- infrared, named for their relation to the visible
spectrum. The higher-energy near-IR, approximately 14000–4000 cm−1 (0.7–2.5 μm wavelength) can excite overtone or harmonic vibrations. The mid-infrared, approximately 4000–400 cm−1 (2.5–25 μm) may be used to study the fundamental vibrations and associated rotational-vibrational structure. The far-infrared, approximately 400–10 cm−1 (25–1000 μm), lying adjacent to the microwave region, has low energy and may be used for rotational spectroscopy.
The names and classifications of these subregions are conventions, and
are only loosely based on the relative molecular or electromagnetic
properties.
Theory
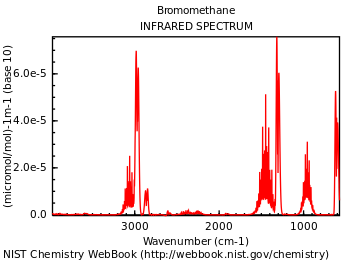
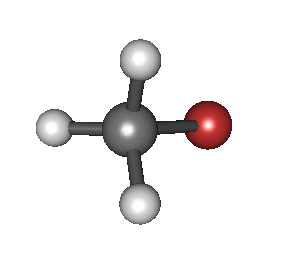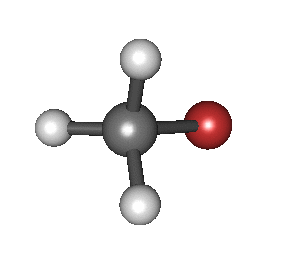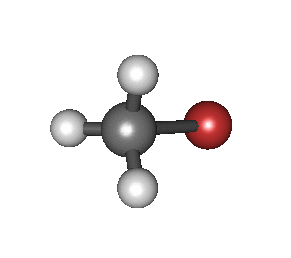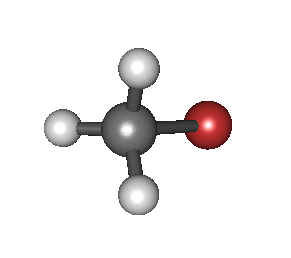
Sample of an IR spec. reading; this one is from bromomethane (CH3Br), showing peaks around 3000, 1300, and 1000 cm−1 (on the horizontal axis).
Infrared spectroscopy exploits the fact that molecules absorb frequencies that are characteristic of their structure. These absorptions occur at resonant frequencies,
i.e. the frequency of the absorbed radiation matches the vibrational
frequency. The energies are affected by the shape of the molecular potential energy surfaces, the masses of the atoms, and the associated vibronic coupling.
3D animation of the symmetric stretching of the C–H bonds of bromomethane
In particular, in the Born–Oppenheimer and harmonic approximations, i.e. when the molecular Hamiltonian corresponding to the electronic ground state can be approximated by a harmonic oscillator in the neighborhood of the equilibrium molecular geometry, the resonant frequencies are associated with the normal modes
corresponding to the molecular electronic ground state potential energy
surface. The resonant frequencies are also related to the strength of
the bond and the mass of the atoms
at either end of it. Thus, the frequency of the vibrations are
associated with a particular normal mode of motion and a particular bond
type.
Number of vibrational modes
In
order for a vibrational mode in a sample to be "IR active", it must be
associated with changes in the dipole moment. A permanent dipole is not
necessary, as the rule requires only a change in dipole moment.
A molecule can vibrate in many ways, and each way is called a vibrational mode.
For molecules with N number of atoms, linear molecules have 3N – 5
degrees of vibrational modes, whereas nonlinear molecules have 3N – 6
degrees of vibrational modes (also called vibrational degrees of
freedom). As an example H2O, a non-linear molecule, will have 3 × 3 – 6 = 3 degrees of vibrational freedom, or modes.
Simple diatomic molecules have only one bond and only one vibrational band. If the molecule is symmetrical, e.g. N2, the band is not observed in the IR spectrum, but only in the Raman spectrum. Asymmetrical diatomic molecules, e.g. CO,
absorb in the IR spectrum. More complex molecules have many bonds, and
their vibrational spectra are correspondingly more complex, i.e. big
molecules have many peaks in their IR spectra.
The atoms in a CH2X2 group, commonly found in organic compounds and where X can represent any other atom, can vibrate in nine different ways. Six of these vibrations involve only the CH2 portion: symmetric and antisymmetric stretching, scissoring, rocking, wagging and twisting,
as shown below. Structures that do not have the two additional X groups
attached have fewer modes because some modes are defined by specific
relationships to those other attached groups. For example, in water, the
rocking, wagging, and twisting modes do not exist because these types
of motions of the H represent simple rotation of the whole molecule
rather than vibrations within it.
| Symmetric | Antisymmetric |
|---|---|
 Symmetric stretching |
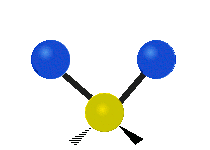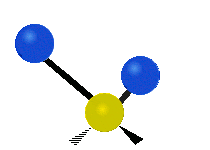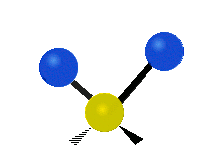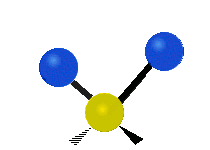 Antisymmetric stretching |
 Scissoring |
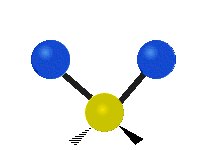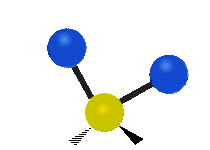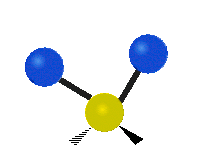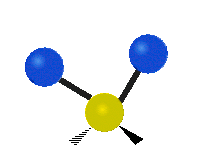 Rocking |
 Wagging |
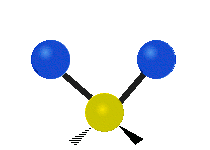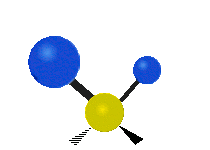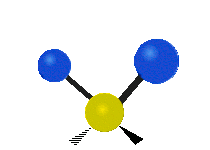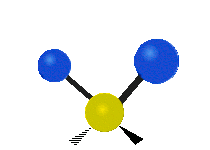 Twisting |
These figures do not represent the "recoil" of the C
atoms, which, though necessarily present to balance the overall
movements of the molecule, are much smaller than the movements of the
lighter H atoms.
Special effects
The simplest and most important or fundamental IR bands arise from the excitations of normal modes, the simplest distortions of the molecule, from the ground state with vibrational quantum number v = 0 to the first excited state with vibrational quantum number v = 1. In some cases, overtone bands
are observed. An overtone band arises from the absorption of a photon
leading to a direct transition from the ground state to the second
excited vibrational state (v = 2). Such a band appears at approximately
twice the energy of the fundamental band for the same normal mode. Some
excitations, so-called combination modes, involve simultaneous excitation of more than one normal mode. The phenomenon of Fermi resonance
can arise when two modes are similar in energy; Fermi resonance results
in an unexpected shift in energy and intensity of the bands etc.
Practical IR spectroscopy
The
infrared spectrum of a sample is recorded by passing a beam of infrared
light through the sample. When the frequency of the IR is the same as
the vibrational frequency of a bond or collection of bonds, absorption
occurs. Examination of the transmitted light reveals how much energy was
absorbed at each frequency (or wavelength). This measurement can be
achieved by scanning the wavelength range using a monochromator. Alternatively, the entire wavelength range is measured using a Fourier transform instrument and then a transmittance or absorbance spectrum is generated using a dedicated procedure.
This technique is commonly used for analyzing samples with covalent bonds.
Simple spectra are obtained from samples with few IR active bonds and
high levels of purity. More complex molecular structures lead to more
absorption bands and more complex spectra.
Typical IR solution cell. The windows are CaF2.
Sample preparation
Gaseous samples require a sample cell with a long pathlength
to compensate for the diluteness. The path length of the sample cell
depends on the concentration of the compound of interest. A simple glass
tube with length of 5 to 10 cm equipped with infrared-transparent
windows at the both ends of the tube can be used for concentrations down
to several hundred ppm. Sample gas concentrations well below ppm can be
measured with a White's cell
in which the infrared light is guided with mirrors to travel through
the gas. White's cells are available with optical path length starting
from 0.5 m up to hundred meters.
Liquid samples can be sandwiched between two plates of a salt (commonly sodium chloride, or common salt, although a number of other salts such as potassium bromide or calcium fluoride are also used).
The plates are transparent to the infrared light and do not introduce any lines onto the spectra.
Solid samples can be prepared in a variety of ways. One common
method is to crush the sample with an oily mulling agent (usually
mineral oil Nujol).
A thin film of the mull is applied onto salt plates and measured. The
second method is to grind a quantity of the sample with a specially
purified salt (usually potassium bromide) finely (to remove scattering effects from large crystals). This powder mixture is then pressed in a mechanical press to form a translucent pellet through which the beam of the spectrometer can pass.
A third technique is the "cast film" technique, which is used mainly
for polymeric materials. The sample is first dissolved in a suitable,
non hygroscopic solvent. A drop of this solution is deposited on surface
of KBr or NaCl cell. The solution is then evaporated to dryness and the
film formed on the cell is analysed directly. Care is important to
ensure that the film is not too thick otherwise light cannot pass
through. This technique is suitable for qualitative analysis. The final
method is to use microtomy
to cut a thin (20–100 µm) film from a solid sample. This is one of the
most important ways of analyzing failed plastic products for example
because the integrity of the solid is preserved.
In photoacoustic spectroscopy
the need for sample treatment is minimal. The sample, liquid or solid,
is placed into the sample cup which is inserted into the photoacoustic
cell which is then sealed for the measurement. The sample may be one
solid piece, powder or basically in any form for the measurement. For
example, a piece of rock can be inserted into the sample cup and the
spectrum measured from it.
Comparing to a reference
Schematics of a two-beam absorption spectrometer. A beam of infrared light is produced, passed through an interferometer
(not shown), and then split into two separate beams. One is passed
through the sample, the other passed through a reference. The beams are
both reflected back towards a detector, however first they pass through a
splitter, which quickly alternates which of the two beams enters the
detector. The two signals are then compared and a printout is obtained.
This "two-beam" setup gives accurate spectra even if the intensity of
the light source drifts over time.
It is typical to record spectrum of both the sample and a "reference". This step controls for a number of variables, e.g. infrared detector, which may affect the spectrum. The reference measurement makes it possible to eliminate the instrument influence.
The appropriate "reference" depends on the measurement and its
goal. The simplest reference measurement is to simply remove the sample
(replacing it by air). However, sometimes a different reference is more
useful. For example, if the sample is a dilute solute dissolved in water
in a beaker, then a good reference measurement might be to measure pure
water in the same beaker. Then the reference measurement would cancel
out not only all the instrumental properties (like what light source is
used), but also the light-absorbing and light-reflecting properties of
the water and beaker, and the final result would just show the
properties of the solute (at least approximately).
A common way to compare to a reference is sequentially: first
measure the reference, then replace the reference by the sample and
measure the sample. This technique is not perfectly reliable; if the
infrared lamp is a bit brighter during the reference measurement, then a
bit dimmer during the sample measurement, the measurement will be
distorted. More elaborate methods, such as a "two-beam" setup (see
figure), can correct for these types of effects to give very accurate
results. The Standard addition method can be used to statistically cancel these errors.
Nevertheless, among different absorption based techniques which are used for gaseous species detection, Cavity ring-down spectroscopy
(CRDS) can be used as a calibration free method. The fact that CRDS is
based on the measurements of photon life-times (and not the laser
intensity) makes it needless for any calibration and comparison with a
reference
FTIR
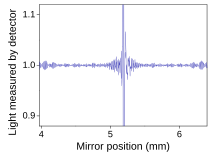
An interferogram from an FTIR
measurement. The horizontal axis is the position of the mirror, and the
vertical axis is the amount of light detected. This is the "raw data"
which can be Fourier transformed to get the actual spectrum.
Fourier transform infrared (FTIR) spectroscopy is a measurement technique that allows one to record infrared spectra. Infrared light is guided through an interferometer
and then through the sample (or vice versa). A moving mirror inside
the apparatus alters the distribution of infrared light that passes
through the interferometer. The signal directly recorded, called an
"interferogram", represents light output as a function of mirror
position. A data-processing technique called Fourier transform turns this raw data into the desired result (the sample's spectrum): Light output as a function of infrared wavelength (or equivalently, wavenumber). As described above, the sample's spectrum is always compared to a reference.
An alternate method for acquiring spectra is the "dispersive" or "scanning monochromator"
method. In this approach, the sample is irradiated sequentially with
various single wavelengths. The dispersive method is more common in UV-Vis spectroscopy, but is less practical in the infrared than the FTIR method. One reason that FTIR is favored is called "Fellgett's advantage" or the "multiplex advantage": The information at all frequencies is collected simultaneously, improving both speed and signal-to-noise ratio.
Another is called "Jacquinot's Throughput Advantage": A dispersive
measurement requires detecting much lower light levels than an FTIR
measurement. There are other advantages, as well as some disadvantages, but virtually all modern infrared spectrometers are FTIR instruments.
Absorption bands
IR spectroscopy is often used to identify structures because functional groups
give rise to characteristic bands both in terms of intensity and
position (frequency). The positions of these bands are summarized in
correlation tables as shown below.
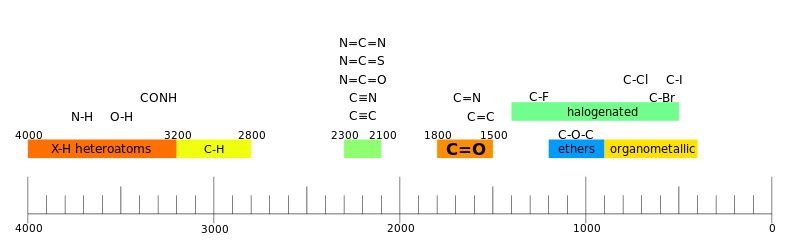
List of main IR spectroscopy bands. For example, the carboxyl group will contain a C = O band at 1700 cm−1 and an OH band at 3500 cm−1 (total group -COOH). Wavenumbers listed in cm−1.
Regions
A spectrograph is often interpreted as having two regions.
- functional group region
In the functional region there are one to a few troughs per functional group.
- fingerprint region
In the fingerprint region there are many troughs which form an
intricate pattern which can be used like a fingerprint to determine the
compound.
Badger's rule
For
many kinds of samples, the assignments are known, i.e. which bond
deformation(s) are associated with which frequency. In such cases
further information can be gleaned about the strength on a bond, relying
on the empirical guideline called Badger's Rule. Originally published by Richard Badger in 1934,
this rule states that the strength of a bond correlates with the
frequency of its vibrational mode. That is, increase in bond strength
leads to corresponding frequency increase and vice versa.
Uses and applications
US Food and Drug Administration scientist uses portable near infrared spectroscopy device to detect potentially illegal substances
Infrared spectroscopy is a simple and reliable technique widely used
in both organic and inorganic chemistry, in research and industry. It is
used in quality control, dynamic measurement, and monitoring
applications such as the long-term unattended measurement of CO2 concentrations in greenhouses and growth chambers by infrared gas analyzers.
It is also used in forensic analysis in both criminal and civil cases, for example in identifying polymer degradation. It can be used in determining the blood alcohol content of a suspected drunk driver.
IR-spectroscopy has been successfully used in analysis and identification of pigments in paintings and other art objects such as illuminated manuscripts.
A useful way of analyzing solid samples without the need for cutting samples uses ATR or attenuated total reflectance
spectroscopy. Using this approach, samples are pressed against the face
of a single crystal. The infrared radiation passes through the crystal
and only interacts with the sample at the interface between the two
materials.
With increasing technology in computer filtering and manipulation
of the results, samples in solution can now be measured accurately
(water produces a broad absorbance across the range of interest, and
thus renders the spectra unreadable without this computer treatment).
Some instruments also automatically identify the substance being
measured from a store of thousands of reference spectra held in storage.
Infrared spectroscopy is also useful in measuring the degree of polymerization in polymer
manufacture. Changes in the character or quantity of a particular bond
are assessed by measuring at a specific frequency over time. Modern
research instruments can take infrared measurements across the range of
interest as frequently as 32 times a second. This can be done whilst
simultaneous measurements are made using other techniques. This makes
the observations of chemical reactions and processes quicker and more
accurate.
Infrared spectroscopy has also been successfully utilized in the field of semiconductor microelectronics: for example, infrared spectroscopy can be applied to semiconductors like silicon, gallium arsenide, gallium nitride, zinc selenide, amorphous silicon, silicon nitride, etc.
Another important application of Infrared Spectroscopy is in the food industry to measure the concentration of various compounds in different food products.
The instruments are now small, and can be transported, even for use in field trials.
Infrared Spectroscopy is also used in gas leak detection devices such as the DP-IR and EyeCGAs. These devices detect hydrocarbon gas leaks in the transportation of natural gas and crude oil.
In February 2014, NASA announced a greatly upgraded database, based on IR spectroscopy, for tracking polycyclic aromatic hydrocarbons (PAHs) in the universe. According to scientists, more than 20% of the carbon in the universe may be associated with PAHs, possible starting materials for the formation of life. PAHs seem to have been formed shortly after the Big Bang, are widespread throughout the universe, and are associated with new stars and exoplanets.
Recent developments include a miniature IR-spectrometer that's
linked to a cloud based database and suitable for personal everyday use, and NIR-spectroscopic chips that can be embedded in smartphones and various gadgets.
Isotope effects
The
different isotopes in a particular species may exhibit different fine
details in infrared spectroscopy. For example, the O–O stretching
frequency (in reciprocal centimeters) of oxyhemocyanin is experimentally determined to be 832 and 788 cm−1 for ν(16O–16O) and ν(18O–18O), respectively.
By considering the O–O bond as a spring, the wavenumber of absorbance, ν can be calculated:
where k is the spring constant for the bond, c is the speed of light, and μ is the reduced mass of the A–B system:
( is the mass of atom ).
The reduced masses for 16O–16O and 18O–18O can be approximated as 8 and 9 respectively. Thus
Where is the wavenumber; [wavenumber = frequency/(speed of light)]
The effect of isotopes, both on the vibration and the decay
dynamics, has been found to be stronger than previously thought. In some
systems, such as silicon and germanium, the decay of the anti-symmetric
stretch mode of interstitial oxygen involves the symmetric stretch mode
with a strong isotope dependence. For example, it was shown that for a
natural silicon sample, the lifetime of the anti-symmetric vibration is
11.4 ps. When the isotope of one of the silicon atoms is increased to 29Si, the lifetime increases to 19 ps. In similar manner, when the silicon atom is changed to 30Si, the lifetime becomes 27 ps.
Two-dimensional IR
Two-dimensional infrared correlation spectroscopy analysis
combines multiple samples of infrared spectra to reveal more complex
properties. By extending the spectral information of a perturbed sample,
spectral analysis is simplified and resolution is enhanced. The 2D
synchronous and 2D asynchronous spectra represent a graphical overview
of the spectral changes due to a perturbation (such as a changing
concentration or changing temperature) as well as the relationship
between the spectral changes at two different wavenumbers.
Pulse Sequence used to obtain a two-dimensional Fourier transform infrared spectrum. The time period is usually referred to as the coherence time and the second time period is known as the waiting time. The excitation frequency is obtained by Fourier transforming along the axis.
Nonlinear two-dimensional infrared spectroscopy is the infrared version of correlation spectroscopy. Nonlinear two-dimensional infrared spectroscopy is a technique that has become available with the development of femtosecond
infrared laser pulses. In this experiment, first a set of pump pulses
is applied to the sample. This is followed by a waiting time during
which the system is allowed to relax. The typical waiting time lasts
from zero to several picoseconds, and the duration can be controlled
with a resolution of tens of femtoseconds. A probe pulse is then
applied, resulting in the emission of a signal from the sample. The
nonlinear two-dimensional infrared spectrum is a two-dimensional
correlation plot of the frequency ω1 that was excited by the initial pump pulses and the frequency ω3
excited by the probe pulse after the waiting time. This allows the
observation of coupling between different vibrational modes; because of
its extremely fine time resolution, it can be used to monitor molecular
dynamics on a picosecond timescale. It is still a largely unexplored
technique and is becoming increasingly popular for fundamental research.
As with two-dimensional nuclear magnetic resonance (2DNMR)
spectroscopy, this technique spreads the spectrum in two dimensions and
allows for the observation of cross peaks that contain information on
the coupling between different modes. In contrast to 2DNMR, nonlinear
two-dimensional infrared spectroscopy also involves the excitation to
overtones. These excitations result in excited state absorption peaks
located below the diagonal and cross peaks. In 2DNMR, two distinct
techniques, COSY and NOESY,
are frequently used. The cross peaks in the first are related to the
scalar coupling, while in the latter they are related to the spin
transfer between different nuclei. In nonlinear two-dimensional infrared
spectroscopy, analogs have been drawn to these 2DNMR techniques.
Nonlinear two-dimensional infrared spectroscopy with zero waiting time
corresponds to COSY, and nonlinear two-dimensional infrared spectroscopy
with finite waiting time allowing vibrational population transfer
corresponds to NOESY. The COSY variant of nonlinear two-dimensional
infrared spectroscopy has been used for determination of the secondary
structure content of proteins.