A Late Silurian sporangium, artificially colored. Green: A spore tetrad. Blue: A spore bearing a trilete mark – the Y-shaped scar. The spores are about 30–35 μm across
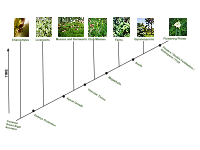
Phylogenetic plant tree, showing the major clades and traditional groups. Monophyletic groups are in black and paraphyletics in blue. Diagram according to symbiogenetic origin of plant cells, and phylogeny of algae, bryophytes, vascular plants, and flowering plants.
The evolution of plants has resulted in a wide range of complexity, from the earliest algal mats, through multicellular marine and freshwater green algae, terrestrial bryophytes, lycopods and ferns, to the complex gymnosperms and angiosperms of today. While many of the earliest groups continue to thrive, as exemplified by red
and green algae in marine environments, more recently derived groups
have displaced previously ecologically dominant ones, e.g. the
ascendance of flowering plants over gymnosperms in terrestrial
environments.
There is evidence that cyanobacteria and multicellular
photosynthetic eukaryotes lived in freshwater communities on land as
early as 1 billion years ago, and that communities of complex, multicellular photosynthesizing organisms existed on land in the late Precambrian, around 850 million years ago.
Evidence of the emergence of embryophyte land plants first occurs in the mid-Ordovician (~470 million years ago), and by the middle of the Devonian (~390 million years ago), many of the features recognized in land plants today were present, including roots and leaves. Late Devonian (~370 million years ago) free-sporing plants such as Archaeopteris had secondary vascular tissue that produced wood and had formed forests of tall trees. Also by late Devonian, Elkinsia, an early seed fern, had evolved seeds.
Evolutionary innovation continued into the Carboniferous and still continues today. Most plant groups were relatively unscathed by the Permo-Triassic extinction event, although the structures of communities changed. This may have set the scene for the appearance of the flowering plants in the Triassic (~200 million years ago), and their later diversification in the Cretaceous and Paleogene. The latest major group of plants to evolve were the grasses, which became important in the mid-Paleogene, from around 40 million years ago. The grasses, as well as many other groups, evolved new mechanisms of metabolism to survive the low CO2 and warm, dry conditions of the tropics over the last 10 million years.
Colonization of land
Land plants evolved from a group of green algae, perhaps as early as 850 mya, but algae-like plants might have evolved as early as 1 billion years ago. The closest living relatives of land plants are the charophytes, specifically Charales;
assuming that the Charales' habit has changed little since the
divergence of lineages, this means that the land plants evolved from a
branched, filamentous alga dwelling in shallow fresh water, perhaps at the edge of seasonally desiccating pools. However, some recent evidence suggests that land plants might have originated from unicellular terrestrial charophytes similar to extant Klebsormidiophyceae. The alga would have had a haplontic life cycle: it would only very briefly have had paired chromosomes (the diploid condition) when the egg and sperm first fused to form a zygote; this would have immediately divided by meiosis to produce cells with half the number of unpaired chromosomes (the haploid condition). Co-operative interactions with fungi may have helped early plants adapt to the stresses of the terrestrial realm.
The
Devonian marks the beginning of extensive land colonization by plants,
which – through their effects on erosion and sedimentation – brought
about significant climatic change.
Cladogram of plant evolution
Plants were not the first photosynthesizers on land. Weathering rates
suggest that organisms capable of photosynthesis were already living on
the land 1,200 million years ago, and microbial fossils have been found in freshwater lake deposits from 1,000 million years ago, but the carbon isotope record suggests that they were too scarce to impact the atmospheric composition until around 850 million years ago. These organisms, although phylogenetically diverse, were probably small and simple, forming little more than an algal scum.
However, evidence of the earliest land plants occurs much later at about 470Ma, in lower middle Ordovician rocks from Saudi Arabia and Gondwana in the form of spores with decay-resistant walls.
These spores, known as cryptospores,
were produced either singly (monads), in pairs (dyads) or groups of
four (tetrads), and their microstructure resembles that of modern liverwort spores, suggesting they share an equivalent grade of organization. Their walls contain sporopollenin – further evidence of an embryophytic affinity. It could be that atmospheric 'poisoning' prevented eukaryotes from colonizing the land prior to this, or it could simply have taken a great time for the necessary complexity to evolve.
Trilete spores similar to those of vascular plants appear soon afterwards, in Upper Ordovician rocks.
Depending exactly when the tetrad splits, each of the four spores may
bear a "trilete mark", a Y-shape, reflecting the points at which each
cell squashed up against its neighbors.
However, this requires that the spore walls be sturdy and resistant at
an early stage. This resistance is closely associated with having a
desiccation-resistant outer wall—a trait only of use when spores must
survive out of water. Indeed, even those embryophytes that have returned to the water lack a resistant wall, thus don't bear trilete marks.
A close examination of algal spores shows that none have trilete
spores, either because their walls are not resistant enough, or in those
rare cases where it is, the spores disperse before they are squashed
enough to develop the mark, or don't fit into a tetrahedral tetrad.
The earliest megafossils of land plants were thalloid
organisms, which dwelt in fluvial wetlands and are found to have
covered most of an early Silurian flood plain. They could only survive
when the land was waterlogged. There were also microbial mats.
Once plants had reached the land, there were two approaches to
dealing with desiccation. Modern bryophytes either avoid it or give in
to it, restricting their ranges to moist settings, or drying out and
putting their metabolism "on hold" until more water arrives, as in the
liverwort genus Targionia. Tracheophytes resist desiccation, by controlling the rate of water loss. They all bear a waterproof outer cuticle
layer wherever they are exposed to air (as do some bryophytes), to
reduce water loss, but since a total covering would cut them off from CO2 in the atmosphere early tracheophytes used variable openings, the stomata,
to regulate the rate of gas exchange. Tracheophytes also developed
vascular tissue to aid in the movement of water within the organisms, and moved away from a gametophyte dominated life cycle.
Vascular tissue ultimately also facilitated upright growth without the
support of water and paved the way for the evolution of larger plants on
land.
A snowball earth,
from around 850-630 mya, is believed to have been caused by early
photosynthetic organisms, which reduced the concentration of carbon
dioxide and increased the amount of oxygen in the atmosphere.
The establishment of a land-based flora increased the rate of
accumulation of oxygen in the atmosphere, as the land plants produced
oxygen as a waste product. When this concentration rose above 13%, wildfires became possible, evident from charcoal in the fossil record. Apart from a controversial gap in the Late Devonian, charcoal is present ever since.
Charcoalification is an important taphonomic
mode. Wildfire or burial in hot volcanic ash drives off the volatile
compounds, leaving only a residue of pure carbon. This is not a viable
food source for fungi, herbivores or detritovores, so is prone to
preservation. It is also robust, so can withstand pressure and display
exquisite, sometimes sub-cellular, detail.
Evolution of life cycles
Angiosperm life cycle
All multicellular plants have a life cycle comprising two generations or phases. The gametophyte phase has a single set of chromosomes (denoted 1n), and produces gametes (sperm and eggs). The sporophyte phase has paired chromosomes (denoted 2n), and produces spores. The gametophyte and sporophyte phases may be homomorphic, appearing identical in some algae, such as Ulva lactuca, but are very different in all modern land plants, a condition known as heteromorphy.
The pattern in plant evolution has been a shift from homomorphy
to heteromorphy. The algal ancestors of land plants were almost
certainly haplobiontic, being haploid for all their life cycles, with a unicellular zygote providing the 2N stage. All land plants (i.e. embryophytes) are diplobiontic – that is, both the haploid and diploid stages are multicellular. Two trends are apparent: bryophytes (liverworts, mosses and hornworts)
have developed the gametophyte as the dominant phase of the life cycle,
with the sporophyte becoming almost entirely dependent on it; vascular plants have developed the sporophyte as the dominant phase, with the gametophytes being particularly reduced in the seed plants.
It has been proposed that the basis for the emergence of the
diploid phase of the life cycle as the dominant phase, is that diploidy
allows masking of the expression of deleterious mutations through genetic complementation. Thus if one of the parental genomes in the diploid cells contains mutations leading to defects in one or more gene products,
these deficiencies could be compensated for by the other parental
genome (which nevertheless may have its own defects in other genes). As
the diploid phase was becoming predominant, the masking effect likely
allowed genome size,
and hence information content, to increase without the constraint of
having to improve accuracy of replication. The opportunity to increase
information content at low cost is advantageous because it permits new
adaptations to be encoded. This view has been challenged, with evidence
showing that selection is no more effective in the haploid than in the
diploid phases of the lifecycle of mosses and angiosperms.
There are two competing theories to explain the appearance of a diplobiontic lifecycle.
The interpolation theory (also known as the antithetic or intercalary theory)
holds that the interpolation of a multicellular sporophyte phase
between two successive gametophyte generations was an innovation caused
by preceding meiosis in a freshly germinated zygote with one or more
rounds of mitotic division, thereby producing some diploid multicellular
tissue before finally meiosis produced spores. This theory implies that
the first sporophytes bore a very different and simpler morphology to
the gametophyte they depended on.
This seems to fit well with what is known of the bryophytes, in which a
vegetative thalloid gametophyte nurtures a simple sporophyte, which
consists of little more than an unbranched sporangium on a stalk.
Increasing complexity of the ancestrally simple sporophyte, including
the eventual acquisition of photosynthetic cells, would free it from its
dependence on a gametophyte, as seen in some hornworts (Anthoceros),
and eventually result in the sporophyte developing organs and vascular
tissue, and becoming the dominant phase, as in the tracheophytes
(vascular plants). This theory may be supported by observations that smaller Cooksonia
individuals must have been supported by a gametophyte generation. The
observed appearance of larger axial sizes, with room for photosynthetic
tissue and thus self-sustainability, provides a possible route for the
development of a self-sufficient sporophyte phase.
The alternative hypothesis, called the transformation theory
(or homologous theory), posits that the sporophyte might have appeared
suddenly by delaying the occurrence of meiosis until a fully developed
multicellular sporophyte had formed. Since the same genetic material
would be employed by both the haploid and diploid phases they would look
the same. This explains the behaviour of some algae, such as Ulva lactuca,
which produce alternating phases of identical sporophytes and
gametophytes. Subsequent adaption to the desiccating land environment,
which makes sexual reproduction difficult, might have resulted in the
simplification of the sexually active gametophyte, and elaboration of
the sporophyte phase to better disperse the waterproof spores. The tissue of sporophytes and gametophytes of vascular plants such as Rhynia preserved in the Rhynie chert is of similar complexity, which is taken to support this hypothesis. By contrast, with the exception of Psilotum
modern vascular plants have heteromorphic sporophytes and gametophytes
in which the gametophytes rarely have any vascular tissue.
Evolution of plant anatomy
Arbuscular mycorrhizal symbiosis
There
is no evidence that early land plants of the Silurian and early
Devonian had roots, although fossil evidence of rhizoids occurs for
several species, such as Horneophyton. The earliest land plants did not have vascular systems for transport of water and nutrients either. Aglaophyton, a rootless vascular plant known from Devonian fossils in the Rhynie chert was the first land plant discovered to have had a mycorrhizal relationship with fungi which formed arbuscules,
literally "tree-like fungal roots", in a well-defined cylinder of cells
(ring in cross section) in the cortex of its stems. The fungi fed on
the plant's sugars, in exchange for nutrients generated or extracted
from the soil (especially phosphate), to which the plant would otherwise
have had no access. Like other rootless land plants of the Silurian and
early Devonian Aglaophyton may have relied on arbuscular mycorrhizal fungi for acquisition of water and nutrients from the soil.
The fungi were of the phylum Glomeromycota,
a group that probably first appeared 1 billion years ago and still
forms arbuscular mycorrhizal associations today with all major land
plant groups from bryophytes to pteridophytes, gymnosperms and
angiosperms and with more than 80% of vascular plants.
Evidence from DNA sequence analysis indicates that the arbuscular
mycorrhizal mutualism arose in the common ancestor of these land plant
groups during their transition to land and it may even have been the critical step that enabled them to colonize the land.
Appearing as they did before these plants had evolved roots,
mycorrhizal fungi would have assisted plants in the acquisition of water
and mineral nutrients such as phosphorus, in exchange for organic compounds which they could not synthesize themselves. Such fungi increase the productivity even of simple plants such as liverworts.
Xylem
To photosynthesize, plants must absorb CO2 from the atmosphere. However, this comes at a price, since making the tissues available for CO2 to enter allows water to evaporate. Water is lost much faster than CO2 is absorbed, so plants need to replace it. Early land plants transported water apoplastically, within the porous walls of their cells. Later, they evolved the ability to control water loss (and CO2 acquisition) through the use of a waterproof outer covering or cuticle perforated by stomata, variable apertures that could open and close to regulate evapotranspiration. Specialised water transport vascular tissues subsequently evolved, first in the form of hydroids, then tracheids and secondary xylem, followed by vessels in flowering plants.
As water transport mechanisms and waterproof cuticles evolved, plants
could survive without being continually covered by a film of water. This
transition from poikilohydry to homoiohydry opened up new potential for colonization.
The high CO2 concentrations of the Silurian and early
Devonian, when plants were first colonizing land, meant that the need
for water was relatively low. As CO2 was withdrawn from the
atmosphere by plants, more water was lost in its capture, and more
elegant water acquisition and transport mechanisms evolved.
Plants then needed a robust internal structure that contained long
narrow channels for transporting water from the soil to all the
different parts of the above-soil plant, especially to the parts where
photosynthesis occurred. By the end of the Carboniferous, when CO2 concentrations had been reduced to something approaching today's, around 17 times more water was lost per unit of CO2 uptake.
However, even in these "easy" early days, water was at a premium, and
had to be transported to parts of the plant from the wet soil to avoid
desiccation. Even today, water transport takes advantage of the cohesion-tension property of water. Water can be wicked
along a fabric with small spaces, and in narrow columns of water, such
as those within the plant cell walls or in tracheids, when molecules
evaporate from one end, they pull the molecules behind them along the
channels. Therefore, transpiration alone provides the driving force for water transport in plants.
However, without dedicated transport vessels, the cohesion-tension
mechanism can cause negative pressures sufficient to collapse the water
conducting cells, limiting the transport water to no more than a few cm,
and therefore limiting the size of the earliest plants.
A banded tube
from the Late Silurian/Early Devonian. The bands are difficult to see
on this specimen, as an opaque carbonaceous coating conceals much of the
tube. Bands are just visible in places on the left half of the image.
Scale bar: 20 μm
To be free from the constraints of small size and constant moisture that the parenchymatic
transport system inflicted, plants needed a more efficient water
transport system. During the early Silurian, they developed specialized
xylem cells, with walls that were strengthened by bands of lignification (or similar chemical compounds). This process was followed by cell death, allowing the cell contents to be emptied and water to be passed through them. These wider, dead, empty cells, the xylem
tracheids, were much more conductive than the inter-cell pathway, and
more resistant to collapse under the tension caused by water stress,
giving the potential for transport over longer distances.
The early Devonian pretracheophytes Aglaophyton and Horneophyton have unreinforced water transport tubes with wall structures very similar to the hydroids of modern moss sporophytes, but they grew alongside several species of tracheophytes, such as Rhynia gwynne-vaughanii
that had well-reinforced xylem tracheids. The earliest macrofossils
known to have xylem tracheids are small, mid-Silurian plants of the
genus Cooksonia.
Plants continued to innovate ways of reducing the resistance to flow
within their cells, thereby increasing the efficiency of their water
transport. Thickened bands on the walls of tubes, apparent from the
early Silurian onwards, are adaptations to increase the resistance to collapse under tension and, when they form single celled conduits, are referred to as tracheids.
These, the "next generation" of transport cell design, have a more
rigid structure than hydroids, preventing their collapse at higher
levels of water tension. Tracheids may have a single evolutionary origin, possibly within the hornworts, uniting all tracheophytes (but they may have evolved more than once).
Water transport requires regulation, and dynamic control is provided by stomata.
By adjusting the rate of gas exchange, they can restrict the amount of
water lost through transpiration. This is an important role where water
supply is not constant, and indeed stomata appear to have evolved before
tracheids, since they are present in the sporophytes of mosses and the
non-vascular hornworts.
An endodermis
may have evolved in the earliest plant roots during the Devonian, but
the first fossil evidence for such a structure is Carboniferous.
The endodermis in the roots surrounds the water transport tissue and
regulates ion exchange (and prevents unwanted pathogens etc. from
entering the water transport system). The endodermis can also provide an
upwards pressure, forcing water out of the roots when transpiration is
not enough of a driver.
Once plants had evolved this level of controlled water transport, they were truly homoiohydric,
able to extract water from their environment through root-like organs
rather than relying on a film of surface moisture, enabling them to grow
to much greater size.
As a result of their independence from their surroundings, they lost
their ability to survive desiccation – a costly trait to retain.
During the Devonian, maximum xylem diameter increased with time, with the minimum diameter remaining pretty constant. By the Middle Devonian, the tracheid diameter of some plant lineages such as the Zosterophyllophytes had plateaued.
Wider tracheids allow water to be transported faster, but the overall
transport rate depends also on the overall cross-sectional area of the
xylem bundle itself.
While wider tracheids with robust walls make it possible to
achieve higher water transport flow rates, this increases the problem of
cavitation
that occurs when the cohesive tension of the water column is broken,
resulting in the formation of a bubble. Pits in tracheid walls have very
small diameters, preventing air bubbles from passing through to
adjacent tracheids., but at the cost of restricted flow rates. By the Carboniferous, Gymnosperms had developed bordered pits, valve-like structures that seal the pits when one side of a tracheid is depressurized.
Growing to height also employed another trait of tracheids – the
support offered by their lignified walls. Defunct tracheids were
retained to form a strong, woody stem, produced in most instances by a
secondary xylem. However, in early plants, tracheids were too
mechanically vulnerable, and retained a central position, with a layer
of tough sclerenchyma on the outer rim of the stems. Even when tracheids do take a structural role, they are supported by sclerenchymatic tissue.
Tracheids end with walls, which impose a great deal of resistance on flow; vessel members have perforated end walls, and are arranged in series to operate as if they were one continuous vessel. The function of end walls, which were the default state in the Devonian, was probably to avoid embolisms.
An embolism is where an air bubble is created in a tracheid. This may
happen as a result of freezing, or by gases dissolving out of solution.
Once an embolism is formed, it usually cannot be removed (but see
later); the affected cell cannot pull water up, and is rendered useless.
End walls excluded, the tracheids of prevascular plants were able
to operate under the same hydraulic conductivity as those of the first
vascular plant, Cooksonia.
The size of tracheids is limited as they comprise a single cell;
this limits their length, which in turn limits their maximum useful
diameter to 80 μm. Conductivity grows with the fourth power of diameter, so increased diameter has huge rewards; vessel elements,
consisting of a number of cells, joined at their ends, overcame this
limit and allowed larger tubes to form, reaching diameters of up to
500 μm, and lengths of up to 10 m.
Vessels first evolved during the dry, low CO2 periods of the Late Permian, in the horsetails, ferns and Selaginellales independently, and later appeared in the mid Cretaceous in angiosperms and gnetophytes.
Vessels allow the same cross-sectional area of wood to transport around a hundred times more water than tracheids! This allowed plants to fill more of their stems with structural fibers, and also opened a new niche to vines, which could transport water without being as thick as the tree they grew on.
Despite these advantages, tracheid-based wood is a lot lighter, thus
cheaper to make, as vessels need to be much more reinforced to avoid
cavitation.
Evolution of plant morphology
Leaves
The lycopod Isoetes bears microphylls (leaves with a single vascular trace).
The branching pattern of megaphyll veins may indicate their origin as webbed, dichotomising branches.
Leaf lamina. The megaphyllous leaf architecture arose multiple times in different plant lineages
Leaves are the primary photosynthetic organs of a modern plant. The origin of leaves was almost certainly triggered by falling concentrations of atmospheric CO2 during the Devonian period, increasing the efficiency with which carbon dioxide could be captured for photosynthesis.
Leaves certainly evolved more than once. Based on their structure, they are classified into two types: microphylls, which lack complex venation and may have originated as spiny outgrowths known as enations, and megaphylls, which are large and have complex venation
that may have arisen from the modification of groups of branches. It
has been proposed that these structures arose independently. Megaphylls, according to Walter Zimmerman's telome theory, have evolved from plants that showed a three-dimensional branching architecture, through three transformations—overtopping, which led to the lateral position typical of leaves, planation, which involved formation of a planar architecture, webbing or fusion, which united the planar branches, thus leading to the formation of a proper leaf lamina. All three steps happened multiple times in the evolution of today's leaves.
It is widely believed that the telome theory is well supported by
fossil evidence. However, Wolfgang Hagemann questioned it for
morphological and ecological reasons and proposed an alternative theory.
Whereas according to the telome theory the most primitive land plants
have a three-dimensional branching system of radially symmetrical axes
(telomes), according to Hagemann's alternative the opposite is proposed:
the most primitive land plants that gave rise to vascular plants were
flat, thalloid, leaf-like, without axes, somewhat like a liverwort or
fern prothallus. Axes such as stems and roots evolved later as new
organs. Rolf Sattler proposed an overarching process-oriented view that
leaves some limited room for both the telome theory and Hagemann's
alternative and in addition takes into consideration the whole continuum
between dorsiventral (flat) and radial (cylindrical) structures that
can be found in fossil and living land plants. This view is supported by research in molecular genetics. Thus, James (2009)
concluded that "it is now widely accepted that... radiality
[characteristic of axes such as stems] and dorsiventrality
[characteristic of leaves] are but extremes of a continuous spectrum. In
fact, it is simply the timing of the KNOX gene expression!"
From the point of view of the telome theory, it has been proposed that before the evolution of leaves, plants had the photosynthetic
apparatus on the stems. Today's megaphyll leaves probably became
commonplace some 360 mya, about 40 my after the simple leafless plants had
colonized the land in the Early Devonian. This spread has been linked to the fall in the atmospheric carbon dioxide concentrations in the Late Paleozoic era associated with a rise in density of stomata on leaf surface. This would have resulted in greater transpiration rates and gas exchange, but especially at high CO2
concentrations, large leaves with fewer stomata would have heated to
lethal temperatures in full sunlight. Increasing the stomatal density
allowed for a better-cooled leaf, thus making its spread feasible, but
increased CO2 uptake at the expense of decreased water use efficiency.
The rhyniophytes of the Rhynie chert consisted of nothing more than slender, unornamented axes. The early to middle Devonian trimerophytes
may be considered leafy. This group of vascular plants are
recognizable by their masses of terminal sporangia, which adorn the ends
of axes which may bifurcate or trifurcate. Some organisms, such as Psilophyton, bore enations. These are small, spiny outgrowths of the stem, lacking their own vascular supply.
Around the same time, the zosterophyllophytes
were becoming important. This group is recognisable by their
kidney-shaped sporangia, which grew on short lateral branches close to
the main axes. They sometimes branched in a distinctive H-shape.
The majority of this group bore pronounced spines on their axes.
However, none of these had a vascular trace, and the first evidence of
vascularised enations occurs in the Rhynie genus Asteroxylon. The spines of Asteroxylon had a primitive vascular supply – at the very least, leaf traces could be seen departing from the central protostele towards each individual "leaf". A fossil clubmoss known as Baragwanathia had already appeared in the fossil record about 20 million years earlier, in the Late Silurian. In this organism, these leaf traces continue into the leaf to form their mid-vein.
One theory, the "enation theory", holds that the leaves developed by
outgrowths of the protostele connecting with existing enations, but it
is also possible that microphylls evolved by a branching axis forming
"webbing".
Asteroxylon and Baragwanathia are widely regarded as primitive lycopods, a group still extant today, represented by the quillworts Isoetes, the spikemosses and the club mosses. Lycopods bear distinctive microphylls, defined as leaves with a single vascular trace. Microphylls could grow to some size, those of Lepidodendrales reaching over a meter in length, but almost all just bear the one vascular bundle. An exception is the rare branching in some Selaginella species.
The more familiar leaves, megaphylls, are thought to have originated four times independently, in the ferns, horsetails, progymnosperms and seed plants. They appear to have originated by modifying dichotomising
branches, which first overlapped (or "overtopped") one another, became
flattened or planated and eventually developed "webbing" and evolved
gradually into more leaf-like structures. Megaphylls, by Zimmerman's telome theory, are composed of a group of webbed branches and hence the "leaf gap" left where the leaf's vascular bundle leaves that of the main branch resembles two axes splitting.
In each of the four groups to evolve megaphylls, their leaves first
evolved during the Late Devonian to Early Carboniferous, diversifying
rapidly until the designs settled down in the mid Carboniferous.
The cessation of further diversification can be attributed to developmental constraints,
but why did it take so long for leaves to evolve in the first place?
Plants had been on the land for at least 50 million years before
megaphylls became significant. However, small, rare mesophylls are known
from the early Devonian genus Eophyllophyton – so development could not have been a barrier to their appearance. The best explanation so far incorporates observations that atmospheric CO2 was declining rapidly during this time – falling by around 90% during the Devonian.
This required an increase in stomatal density by 100 times to maintain
rates of photosynthesis. When stomata open to allow water to evaporate
from leaves it has a cooling effect, resulting from the loss of latent heat
of evaporation. It appears that the low stomatal density in the early
Devonian meant that evaporation and evaporative cooling were limited,
and that leaves would have overheated if they grew to any size. The
stomatal density could not increase, as the primitive steles and limited
root systems would not be able to supply water quickly enough to match
the rate of transpiration.
Clearly, leaves are not always beneficial, as illustrated by the
frequent occurrence of secondary loss of leaves, famously exemplified by
cacti and the "whisk fern" Psilotum.
Secondary evolution can also disguise the true evolutionary
origin of some leaves. Some genera of ferns display complex leaves which
are attached to the pseudostele by an outgrowth of the vascular bundle,
leaving no leaf gap. Further, horsetail (Equisetum)
leaves bear only a single vein, and appear to be microphyllous;
however, both the fossil record and molecular evidence indicate that
their forebears bore leaves with complex venation, and the current state
is a result of secondary simplification.
Deciduous
trees deal with another disadvantage to having leaves. The popular
belief that plants shed their leaves when the days get too short is
misguided; evergreens prospered in the Arctic circle during the most recent greenhouse earth.
The generally accepted reason for shedding leaves during winter is to
cope with the weather – the force of wind and weight of snow are much
more comfortably weathered without leaves to increase surface area.
Seasonal leaf loss has evolved independently several times and is
exhibited in the ginkgoales, some pinophyta and certain angiosperms.
Leaf loss may also have arisen as a response to pressure from insects;
it may have been less costly to lose leaves entirely during the winter
or dry season than to continue investing resources in their repair.
Factors influencing leaf architectures
Various physical and physiological factors such as light intensity, humidity, temperature, wind speeds
etc. have influenced evolution of leaf shape and size. High trees
rarely have large leaves, because they are damaged by high winds.
Similarly, trees that grow in temperate or taiga regions have pointed leaves, presumably to prevent nucleation of ice onto the leaf surface and reduce water loss due to transpiration. Herbivory, by mammals and insects, has been a driving force in leaf evolution. An example is that plants of the New Zealand genus Aciphylla have spines on their laminas, which probably functioned to discourage the extinct Moas from feeding on them. Other members of Aciphylla, which did not co-exist with the moas, do not have these spines.
At the genetic level, developmental studies have shown that repression of KNOX genes is required for initiation of the leaf primordium. This is brought about by ARP genes, which encode transcription factors.
Repression of KNOX genes in leaf primordia seems to be quite conserved,
while expression of KNOX genes in leaves produces complex leaves. The ARP function appears to have arisen early in vascular plant evolution, because members of the primitive group Lycophytes also have a functionally similar gene. Other players that have a conserved role in defining leaf primordia are the phytohormones auxin, gibberelin and cytokinin.
The diversity of leaves
The arrangement of leaves or phyllotaxy on the plant body can maximally harvest light and might be expected to be genetically robust. However, in maize, a mutation in only one gene called ABPHYL (ABnormal PHYLlotaxy) is enough to change the phyllotaxy of the leaves, implying that mutational adjustment of a single locus on the genome is enough to generate diversity.
Once the leaf primordial cells are established from the SAM cells, the new axes
for leaf growth are defined, among them being the abaxial-adaxial
(lower-upper surface) axes. The genes involved in defining this, and the
other axes seem to be more or less conserved among higher plants.
Proteins of the HD-ZIPIII family have been implicated in defining the adaxial identity. These proteins deviate some cells in the leaf primordium from the default abaxial state, and make them adaxial.
In early plants with leaves, the leaves probably just had one type of
surface — the abaxial one, the underside of today's leaves. The
definition of the adaxial identity occurred some 200 million years after
the abaxial identity was established.
How the wide variety of observed plant leaf morphology is
generated is a subject of intense research. Some common themes have
emerged. One of the most significant is the involvement of KNOX genes in
generating compound leaves, as in the tomato. But, this is not universal. For example, the pea uses a different mechanism for doing the same thing. Mutations in genes affecting leaf curvature can also change leaf form, by changing the leaf from flat, to a crinkly shape, like the shape of cabbage leaves. There also exist different morphogen
gradients in a developing leaf which define the leaf's axis and may
also affect the leaf form. Another class of regulators of leaf
development are the micro RNAs.
Roots
Roots are important to plants for two main reasons: Firstly, they provide anchorage to the substrate; more importantly, they provide a source of water and nutrients from the soil. Roots allowed plants to grow taller and faster.
The evolution of roots had consequences on a global scale. By
disturbing the soil and promoting its acidification (by taking up
nutrients such as nitrate and phosphate), they enabled it to weather more deeply, injecting carbon compounds deeper into soils with huge implications for climate. These effects may have been so profound they led to a mass extinction.
While there are traces of root-like impressions in fossil soils in the Late Silurian,
body fossils show the earliest plants to be devoid of roots. Many had
prostrate branches that sprawled along the ground, with upright axes or thalli
dotted here and there, and some even had non-photosynthetic
subterranean branches which lacked stomata. The distinction between root
and specialized branch is developmental. differing in their branching pattern and in possession of a root cap. So while Siluro-Devonian plants such as Rhynia and Horneophyton possessed the physiological equivalent of roots, roots – defined as organs differentiated from stems – did not arrive until later. Unfortunately, roots are rarely preserved in the fossil record, and our understanding of their evolutionary origin is sparse.
Rhizoids – small structures performing the same role as roots,
usually a cell in diameter – probably evolved very early, perhaps even
before plants colonized the land; they are recognized in the Characeae, an algal sister group to land plants. That said, rhizoids probably evolved more than once; the rhizines of lichens, for example, perform a similar role. Even some animals (Lamellibrachia) have root-like structures.
Rhizoids are clearly visible in the Rhynie chert fossils, and were
present in most of the earliest vascular plants, and on this basis seem
to have presaged true plant roots.
More advanced structures are common in the Rhynie chert, and many
other fossils of comparable early Devonian age bear structures that
look like, and acted like, roots.
The rhyniophytes bore fine rhizoids, and the trimerophytes and
herbaceous lycopods of the chert bore root-like structure penetrating a
few centimeters into the soil. However, none of these fossils display all the features borne by modern roots, with the exception of Asteroxylon, which has recently been recognized as bearing roots that evolved independently from those of extant vascular plants. Roots and root-like structures became increasingly common and deeper penetrating during the Devonian,
with lycopod trees forming roots around 20 cm long during the Eifelian
and Givetian. These were joined by progymnosperms, which rooted up to
about a metre deep, during the ensuing Frasnian stage. True gymnosperms and zygopterid ferns also formed shallow rooting systems during the Famennian.
The rhizophores of the lycopods provide a slightly different
approach to rooting. They were equivalent to stems, with organs
equivalent to leaves performing the role of rootlets. A similar construction is observed in the extant lycopod Isoetes, and this appears to be evidence that roots evolved independently at least twice, in the lycophytes and other plants,
a proposition supported by studies showing that roots are initiated and
their growth promoted by different mechanisms in lycophytes and
euphyllophytes.
A vascular system is indispensable to rooted plants, as
non-photosynthesising roots need a supply of sugars, and a vascular
system is required to transport water and nutrients from the roots to
the rest of the plant. Rooted plants
are little more advanced than their Silurian forebears, without a
dedicated root system; however, the flat-lying axes can be clearly seen
to have growths similar to the rhizoids of bryophytes today.
By the Middle to Late Devonian, most groups of plants had independently developed a rooting system of some nature. As roots became larger, they could support larger trees, and the soil was weathered to a greater depth. This deeper weathering had effects not only on the aforementioned drawdown of CO2, but also opened up new habitats for colonisation by fungi and animals.
Roots today have developed to the physical limits. They penetrate as much as 60 metres of soil to tap the water table. The narrowest roots are a mere 40 μm in diameter, and could not physically transport water if they were any narrower.
The earliest fossil roots recovered, by contrast, narrowed from 3 mm to under 700 μm in diameter; of course, taphonomy is the ultimate control of what thickness can be seen.
Tree form
The trunk of early tree fern Psaronius, showing internal structure. The top of the plant would have been to the left of the image
External mold of Lepidodendron trunk showing leaf scars from the Upper Carboniferous of Ohio
The early Devonian landscape was devoid of vegetation taller than
waist height. Greater height provided a competitive advantage in the
harvesting of sunlight for photosynthesis, overshadowing of
competitors and in spore distribution, as spores (and later, seeds)
could be blown for greater distances if they started higher. An
effective vascular system was required in order to achieve greater
heights. To attain arborescence, plants had to develop woody tissue that provided both support and water transport, and thus needed to evolve the capacity for secondary growth. The stele of plants undergoing secondary growth is surrounded by a vascular cambium,
a ring of meristematic cells which produces more xylem on the inside
and phloem on the outside. Since xylem cells comprise dead, lignified
tissue, subsequent rings of xylem are added to those already present,
forming wood.
The first plants to develop secondary growth and a woody habit,
were apparently the ferns, and as early as the Middle Devonian one
species, Wattieza, had already reached heights of 8 m and a tree-like habit.
Other clades did not take long to develop a tree-like stature. The Late Devonian Archaeopteris, a precursor to gymnosperms which evolved from the trimerophytes,
reached 30 m in height. The progymnosperms were the first plants to
develop true wood, grown from a bifacial cambium. The first appearance
of one of them, Rellimia, was in the Middle Devonian. True wood is only thought to have evolved once, giving rise to the concept of a "lignophyte" clade.
Archaeopteris forests were soon supplemented by arborescent lycopods, in the form of Lepidodendrales,
which exceeded 50m in height and 2m across at the base. These
arborescent lycopods rose to dominate Late Devonian and Carboniferous
forests that gave rise to coal deposits.
Lepidodendrales differ from modern trees in exhibiting determinate
growth: after building up a reserve of nutrients at a lower height, the
plants would "bolt" as a single trunk to a genetically determined
height, branch at that level, spread their spores and die. They consisted of "cheap" wood to allow their rapid growth, with at least half of their stems comprising a pith-filled cavity.
Their wood was also generated by a unifacial vascular cambium – it did
not produce new phloem, meaning that the trunks could not grow wider
over time.
The horsetail Calamites appeared in the Carboniferous. Unlike the modern horsetail Equisetum, Calamites had a unifacial vascular cambium, allowing them to develop wood and grow to heights in excess of 10 m and to branch repeatedly.
While the form of early trees was similar to that of today's, the Spermatophytes
or seed plants, the group that contain all modern trees, had yet to
evolve. The dominant tree groups today are all seed plants, the
gymnosperms, which include the coniferous trees, and the angiosperms,
which contain all fruiting and flowering trees. No free-sporing trees
like Archaeopteris exist in the extant flora. It was long
thought that the angiosperms arose from within the gymnosperms, but
recent molecular evidence suggests that their living representatives
form two distinct groups. The molecular data has yet to be fully reconciled with morphological data, but it is becoming accepted that the morphological support for paraphyly is not especially strong.
This would lead to the conclusion that both groups arose from within the pteridosperms, probably as early as the Permian.
The angiosperms and their ancestors played a very small role
until they diversified during the Cretaceous. They started out as small,
damp-loving organisms in the understorey, and have been diversifying
ever since the mid-Cretaceous, to become the dominant member of non-boreal forests today.
Seeds
The fossil seed Trigonocarpus
The transitional fossil Runcaria
Early land plants reproduced in the fashion of ferns: spores
germinated into small gametophytes, which produced eggs and/or sperm.
These sperm would swim across moist soils to find the female organs
(archegonia) on the same or another gametophyte, where they would fuse
with an egg to produce an embryo, which would germinate into a
sporophyte.
Heterosporic plants, as their name suggests, bear spores of two
sizes – microspores and megaspores. These would germinate to form
microgametophytes and megagametophytes, respectively. This system paved
the way for ovules and seeds: taken to the extreme, the megasporangia
could bear only a single megaspore tetrad, and to complete the
transition to true ovules, three of the megaspores in the original
tetrad could be aborted, leaving one megaspore per megasporangium.
The transition to ovules continued with this megaspore being
"boxed in" to its sporangium while it germinates. Then, the
megagametophyte is contained within a waterproof integument, which forms
the bulk of the seed. The microgametophyte – a pollen grain which has
germinated from a microspore – is employed for dispersal, only releasing
its desiccation-prone sperm when it reaches a receptive
megagametophyte.
Lycopods and sphenopsids got a fair way down the path to the seed
habit without ever crossing the threshold. Fossil lycopod megaspores
reaching 1 cm in diameter, and surrounded by vegetative tissue, are
known (Lepidocarpon, Achlamydocarpon);– these even germinate into a
megagametophyte in situ. However, they fall short of being
ovules, since the nucellus, an inner spore-covering layer, does not
completely enclose the spore. A very small slit (micropyle) remains,
meaning that the megasporangium is still exposed to the atmosphere. This
has two consequences – firstly, it means it is not fully resistant to
desiccation, and secondly, sperm do not have to "burrow" to access the
archegonia of the megaspore.
A Middle Devonian precursor to seed plants from Belgium has been identified predating the earliest seed plants by about 20 million years. Runcaria, small and radially symmetrical, is an integumented megasporangium surrounded by a cupule. The megasporangium bears an unopened distal extension protruding above the multilobed integument. It is suspected that the extension was involved in anemophilous pollination. Runcaria sheds new light on the sequence of character acquisition leading to the seed. Runcaria has all of the qualities of seed plants except for a solid seed coat and a system to guide the pollen to the ovule.
The first spermatophytes (literally: "seed plants") – that is, the first plants to bear true seeds – are called pteridosperms:
literally, "seed ferns", so called because their foliage consisted of
fern-like fronds, although they were not closely related to ferns. The
oldest fossil evidence of seed plants is of Late Devonian age, and they
appear to have evolved out of an earlier group known as the progymnosperms.
These early seed plants ranged from trees to small, rambling shrubs;
like most early progymnosperms, they were woody plants with fern-like
foliage. They all bore ovules, but no cones, fruit or similar. While it
is difficult to track the early evolution of seeds, the lineage of the
seed ferns may be traced from the simple trimerophytes through
homosporous Aneurophytes.
This seed model is shared by basically all gymnosperms
(literally: "naked seeds"), most of which encase their seeds in a woody
cone or fleshy aril (the yew,
for example), but none of which fully enclose their seeds. The
angiosperms ("vessel seeds") are the only group to fully enclose the
seed, in a carpel.
Fully enclosed seeds opened up a new pathway for plants to follow: that of seed dormancy.
The embryo, completely isolated from the external atmosphere and hence
protected from desiccation, could survive some years of drought before
germinating.
Gymnosperm seeds from the Late Carboniferous have been found to contain
embryos, suggesting a lengthy gap between fertilisation and germination. This period is associated with the entry into a greenhouse earth
period, with an associated increase in aridity. This suggests that
dormancy arose as a response to drier climatic conditions, where it
became advantageous to wait for a moist period before germinating.
This evolutionary breakthrough appears to have opened a floodgate:
previously inhospitable areas, such as dry mountain slopes, could now be
tolerated, and were soon covered by trees.
Seeds offered further advantages to their bearers: they increased
the success rate of fertilised gametophytes, and because a nutrient
store could be "packaged" in with the embryo, the seeds could germinate
rapidly in inhospitable environments, reaching a size where it could
fend for itself more quickly.
For example, without an endosperm, seedlings growing in arid
environments would not have the reserves to grow roots deep enough to
reach the water table before they expired from dehydration.
Likewise, seeds germinating in a gloomy understory require an
additional reserve of energy to quickly grow high enough to capture
sufficient light for self-sustenance.
A combination of these advantages gave seed plants the ecological edge over the previously dominant genus Archaeopteris, thus increasing the biodiversity of early forests.
Despite these advantages, it is common for fertilized ovules to fail to mature as seeds. Also during seed dormancy (often associated with unpredictable and stressful conditions) DNA damage accumulates. Thus DNA damage appears to be a basic problem for survival of seed plants, just as DNA damage is a major problem for life in general.
Flowers
The pollen bearing organs of the early "flower" Crossotheca
Flowers are modified leaves possessed only by the angiosperms,
which are relatively late to appear in the fossil record. The group
originated and diversified during the Early Cretaceous and became
ecologically significant thereafter. Flower-like structures first appear in the fossil records some ~130 mya, in the Cretaceous. However, more recently, in 2018, scientists reported that the earliest flowers began about 180 million years ago, 50 million years earlier than thought earlier.
Colorful and/or pungent structures surround the cones of plants such as cycads and Gnetales, making a strict definition of the term "flower" elusive.
The main function of a flower is reproduction, which, before the evolution of the flower and angiosperms, was the job of microsporophylls and megasporophylls. A flower can be considered a powerful evolutionary innovation, because its presence allowed the plant world to access new means and mechanisms for reproduction.
The evolution of syncarps.
a: sporangia borne at tips of leaf
b: Leaf curls up to protect sporangia
c: leaf curls to form enclosed roll
d: grouping of three rolls into a syncarp
a: sporangia borne at tips of leaf
b: Leaf curls up to protect sporangia
c: leaf curls to form enclosed roll
d: grouping of three rolls into a syncarp
The flowering plants have long been assumed to have evolved from within the gymnosperms; according to the traditional morphological view, they are closely allied to the Gnetales. However, as noted above, recent molecular evidence is at odds with this hypothesis, and further suggests that Gnetales are more closely related to some gymnosperm groups than angiosperms, and that extant gymnosperms form a distinct clade to the angiosperms, the two clades diverging some 300 million years ago.
The relationship of stem groups to the angiosperms
is important in determining the evolution of flowers. Stem groups
provide an insight into the state of earlier "forks" on the path to the
current state. Convergence increases the risk of misidentifying stem
groups. Since the protection of the megagametophyte
is evolutionarily desirable, probably many separate groups evolved
protective encasements independently. In flowers, this protection takes
the form of a carpel,
evolved from a leaf and recruited into a protective role, shielding the
ovules. These ovules are further protected by a double-walled integument.
Penetration of these protective layers needs something more than a free-floating microgametophyte. Angiosperms
have pollen grains comprising just three cells. One cell is responsible
for drilling down through the integuments, and creating a conduit for
the two sperm cells to flow down. The megagametophyte has just seven
cells; of these, one fuses with a sperm cell, forming the nucleus of the
egg itself, and another joins with the other sperm, and dedicates
itself to forming a nutrient-rich endosperm. The other cells take auxiliary roles. This process of "double fertilization" is unique and common to all angiosperms.
The inflorescences of the Bennettitales are strikingly similar to flowers
In the fossil record, there are three intriguing groups which bore flower-like structures. The first is the Permian pteridosperm Glossopteris, which already bore recurved leaves resembling carpels. The Mesozoic Caytonia
is more flower-like still, with enclosed ovules – but only a single
integument. Further, details of their pollen and stamens set them apart
from true flowering plants.
The Bennettitales bore remarkably flower-like organs, protected by whorls of bracts
which may have played a similar role to the petals and sepals of true
flowers; however, these flower-like structures evolved independently, as
the Bennettitales are more closely related to cycads and ginkgos than to the angiosperms.
However, no true flowers are found in any groups save those extant today. Most morphological and molecular analyses place Amborella, the nymphaeales and Austrobaileyaceae in a basal clade called "ANA". This clade appear to have diverged in the early Cretaceous, around 130 million years ago – around the same time as the earliest fossil angiosperm, and just after the first angiosperm-like pollen, 136 million years ago.
The magnoliids diverged soon after, and a rapid radiation had produced eudicots and monocots by 125 million years ago. By the end of the Cretaceous 66 million years ago, over 50% of today's angiosperm orders had evolved, and the clade accounted for 70% of global species.
It was around this time that flowering trees became dominant over conifers.
The features of the basal "ANA" groups suggest that angiosperms originated in dark, damp, frequently disturbed areas.
It appears that the angiosperms remained constrained to such habitats
throughout the Cretaceous – occupying the niche of small herbs early in
the successional series.
This may have restricted their initial significance, but given them the
flexibility that accounted for the rapidity of their later
diversifications in other habitats.
Some propose that the Angiosperms arose from an unknown Seed Fern,
Pteridophyte, and view Cycads as living Seed Ferns with both
Seed-Bearing and sterile leaves (Cycas revoluta).
In August 2017, scientists presented a detailed description and 3D image of a reconstruction of possibly the first flower that lived about 140 million years ago.
Origins of the flower
A male flower of Amborella trichopoda. Amborellaceae is considered the sister family of all other flowering plants.
The family Amborellaceae is regarded as being the sister clade to all other living flowering plants. The complete genome of Amborella trichopoda is still being sequenced as of March 2012.
By comparing its genome with those of all other living flowering
plants, it will be possible to work out the most likely characteristics
of the ancestor of A. trichopoda and all other flowering plants, i.e. the ancestral flowering plant.
It seems that on the level of the organ, the leaf may be the ancestor of the flower, or at least some floral organs. When some crucial genes involved in flower development are mutated,
clusters of leaf-like structures arise in place of flowers. Thus,
sometime in history, the developmental program leading to formation of a
leaf must have been altered to generate a flower. There probably also
exists an overall robust framework within which the floral diversity has
been generated. An example of that is a gene called LEAFY (LFY), which is involved in flower development in Arabidopsis thaliana. The homologs of this gene are found in angiosperms as diverse as tomato, snapdragon, pea, maize and even gymnosperms. Expression of Arabidopsis thaliana LFY in distant plants like poplar and citrus also results in flower-production in these plants. The LFY gene regulates the expression of some genes belonging to the MADS-box family. These genes, in turn, act as direct controllers of flower development.
Evolution of the MADS-box family
The members of the MADS-box
family of transcription factors play a very important and
evolutionarily conserved role in flower development. According to the ABC Model of flower development, three zones — A, B and C — are generated within the developing flower primordium, by the action of some transcription factors, that are members of the MADS-box
family. Among these, the functions of the B and C domain genes have
been evolutionarily more conserved than the A domain gene. Many of these
genes have arisen through gene duplications of ancestral members of this family. Quite a few of them show redundant functions.
The evolution of the MADS-box family has been extensively studied. These genes are present even in pteridophytes, but the spread and diversity is many times higher in angiosperms. There appears to be quite a bit of pattern into how this family has evolved. Consider the evolution of the C-region gene AGAMOUS (AG). It is expressed in today's flowers in the stamens, and the carpel, which are reproductive organs. Its ancestor in gymnosperms also has the same expression pattern. Here, it is expressed in the strobili, an organ that produces pollen or ovules. Similarly, the B-genes' (AP3 and PI) ancestors are expressed only in the male organs in gymnosperms. Their descendants in the modern angiosperms also are expressed only in the stamens,
the male reproductive organ. Thus, the same, then-existing components
were used by the plants in a novel manner to generate the first flower.
This is a recurring pattern in evolution.
Factors influencing floral diversity
There is enormous variation in floral structure in plants, typically due to changes in the MADS-box
genes and their expression pattern. For example, grasses possess unique
floral structures. The carpels and stamens are surrounded by scale-like
lodicules and two bracts, the lemma and the palea, but genetic evidence and morphology suggest that lodicules are homologous to eudicot petals. The palea and lemma may be homologous to sepals in other groups, or may be unique grass structures.
Another example is that of Linaria vulgaris, which has two kinds of flower symmetries-radial and bilateral. These symmetries are due to epigenetic changes in just one gene called CYCLOIDEA.
Arabidopsis thaliana has a gene called AGAMOUS that plays an important role in defining how many petals and sepals and other organs are generated. Mutations in this gene give rise to the floral meristem obtaining an indeterminate fate, and proliferation of floral organs in double-flowered forms of roses, carnations and morning glory. These phenotypes have been selected by horticulturists for their increased number of petals. Several studies on diverse plants like petunia, tomato, Impatiens, maize etc. have suggested that the enormous diversity of flowers is a result of small changes in genes controlling their development.
The Floral Genome Project confirmed that the ABC Model of flower development is not conserved across all angiosperms. Sometimes expression domains change, as in the case of many monocots, and also in some basal angiosperms like Amborella. Different models of flower development like the Fading boundaries model, or the Overlapping-boundaries model which propose non-rigid domains of expression, may explain these architectures.
There is a possibility that from the basal to the modern angiosperms,
the domains of floral architecture have become more and more fixed
through evolution.
Flowering time
Another floral feature that has been a subject of natural selection is flowering time. Some plants flower early in their life cycle, others require a period of vernalization before flowering. This outcome is based on factors like temperature, light intensity, presence of pollinators and other environmental signals: genes like CONSTANS (CO), Flowering Locus C (FLC) and FRIGIDA
regulate integration of environmental signals into the pathway for
flower development. Variations in these loci have been associated with
flowering time variations between plants. For example, Arabidopsis thaliana ecotypes that grow in the cold, temperate regions require prolonged vernalization before they flower, while the tropical varieties, and the most common lab strains, don't. This variation is due to mutations in the FLC and FRIGIDA genes, rendering them non-functional.
Many of the genes involved in this process are conserved across
all the plants studied. Sometimes though, despite genetic conservation,
the mechanism of action turns out to be different. For example, rice is a short-day plant, while Arabidopsis thaliana is a long-day plant. Both plants have the proteins CO and FLOWERING LOCUS T (FT), but, in Arabidopsis thaliana, CO enhances FT production, while in rice, the CO homolog represses FT production, resulting in completely opposite downstream effects.
Theories of flower evolution
The Anthophyte theory was based on the observation that a gymnospermic group Gnetales has a flower-like ovule. It has partially developed vessels as found in the angiosperms, and the megasporangium is covered by three envelopes, like the ovary structure of angiosperm flowers. However, many other lines of evidence show that Gnetales is not related to angiosperms.
The Mostly Male theory has a more genetic basis. Proponents of this theory point out that the gymnosperms have two very similar copies of the gene LFY, while angiosperms just have one. Molecular clock analysis has shown that the other LFY
paralog was lost in angiosperms around the same time as flower fossils
become abundant, suggesting that this event might have led to floral
evolution. According to this theory, loss of one of the LFY paralog led to flowers that were more male, with the ovules being expressed ectopically. These ovules initially performed the function of attracting pollinators, but sometime later, may have been integrated into the core flower.
Mechanisms and players in evolution of plant morphology
The stem-loop secondary structure of a pre-micro RNA from Brassica oleracea
While environmental factors are significantly responsible for evolutionary change, they act merely as agents for natural selection. Change is inherently brought about via phenomena at the genetic level: mutations, chromosomal rearrangements, and epigenetic changes. While the general types of mutations hold true across the living world, in plants, some other mechanisms have been implicated as highly significant.
Genome doubling is a relatively common occurrence in plant evolution and results in polyploidy,
which is consequently a common feature in plants. It is estimated that
at least half (and probably all) plants have seen genome doubling in
their history. Genome doubling entails gene duplication,
thus generating functional redundancy in most genes. The duplicated
genes may attain new function, either by changes in expression pattern
or changes in activity. Polyploidy and gene duplication are believed to
be among the most powerful forces in evolution of plant form; though it
is not known why genome doubling is such a frequent process in plants. One probable reason is the production of large amounts of secondary metabolites in plant cells. Some of them might interfere in the normal process of chromosomal segregation, causing genome duplication.
In recent times, plants have been shown to possess significant microRNA families, which are conserved across many plant lineages. In comparison to animals, while the number of plant miRNA families are lesser than animals, the size of each family is much larger. The miRNA
genes are also much more spread out in the genome than those in
animals, where they are more clustered. It has been proposed that these
miRNA families have expanded by duplications of chromosomal regions. Many miRNA genes involved in regulation of plant development have been found to be quite conserved between plants studied.
Domestication of plants like maize, rice, barley, wheat
etc. has also been a significant driving force in their evolution.
Research concerning the origin of maize has found that it is a
domesticated derivative of a wild plant from Mexico called teosinte. Teosinte belongs to the genus Zea, just as maize, but bears very small inflorescence, 5–10 hard cobs and a highly branched and spread out stem.
Cauliflower – Brassica oleracea var. botrytis
Crosses between a particular teosinte variety and maize yields fertile offspring that are intermediate in phenotype between maize and teosinte. QTL analysis has also revealed some loci that, when mutated in maize, yield a teosinte-like stem or teosinte-like cobs. Molecular clock
analysis of these genes estimates their origins to some 9,000 years
ago, well in accordance with other records of maize domestication. It is
believed that a small group of farmers must have selected some
maize-like natural mutant of teosinte some 9,000 years ago in Mexico,
and subjected it to continuous selection to yield the familiar maize
plant of today.
The edible cauliflower is a domesticated version of the wild plant Brassica oleracea, which does not possess the dense undifferentiated inflorescence, called the curd, that cauliflower possesses.
Cauliflower possesses a single mutation in a gene called CAL, controlling meristem differentiation into inflorescence. This causes the cells at the floral meristem to gain an undifferentiated identity and, instead of growing into a flower, they grow into a dense mass of inflorescence meristem cells in arrested development. This mutation has been selected through domestication since at least the time of the Greek empire.
Evolution of photosynthetic pathways
The C4 carbon concentrating mechanism
The C4 metabolic pathway is a valuable recent evolutionary innovation in plants, involving a complex set of adaptive changes to physiology and gene expression patterns.
Photosynthesis is a complex chemical pathway facilitated by a range of enzymes and co-enzymes. The enzyme RuBisCO is responsible for "fixing" CO2 –
that is, it attaches it to a carbon-based molecule to form a sugar,
which can be used by the plant, releasing an oxygen molecule. However,
the enzyme is notoriously inefficient, and, as ambient temperature
rises, will increasingly fix oxygen instead of CO2 in a process called photorespiration.
This is energetically costly as the plant has to use energy to turn the
products of photorespiration back into a form that can react with CO2.
Concentrating carbon
C4 plants evolved carbon concentrating mechanisms that work by increasing the concentration of CO2
around RuBisCO, and excluding oxygen, thereby increasing the efficiency
of photosynthesis by decreasing photorespiration. The process of
concentrating CO2 around RuBisCO requires more energy than allowing gases to diffuse, but under certain conditions – i.e. warm temperatures (greater than 25 °C), low CO2 concentrations, or high oxygen concentrations – pays off in terms of the decreased loss of sugars through photorespiration.
One type of C4 metabolism employs a so-called Kranz anatomy. This transports CO2 through an outer mesophyll layer, via a range of organic molecules, to the central bundle sheath cells, where the CO2 is released. In this way, CO2 is concentrated near the site of RuBisCO operation. Because RuBisCO is operating in an environment with much more CO2 than it otherwise would be, it performs more efficiently.
A second mechanism, CAM photosynthesis,
temporally separates photosynthesis from the action of RuBisCO. RuBisCO
only operates during the day, when stomata are sealed and CO2 is provided by the breakdown of the chemical malate. More CO2 is then harvested from the atmosphere when stomata open, during the cool, moist nights, reducing water loss.
Evolutionary record
These two pathways, with the same effect on RuBisCO, evolved a number of times independently – indeed, C4 alone arose 62 times in 18 different plant families.
A number of 'pre-adaptations' seem to have paved the way for C4,
leading to its clustering in certain clades: it has most frequently been
innovated in plants that already had features such as extensive
vascular bundle sheath tissue. Many potential evolutionary pathways resulting in the C4 phenotype are possible and have been characterized using Bayesian inference, confirming that non-photosynthetic adaptations often provide evolutionary stepping stones for the further evolution of C4.
The C4 construction is used by a subset of grasses, while CAM is employed by many succulents and cacti. The C4 trait appears to have emerged during the Oligocene, around 25 to 32 million years ago; however, they did not become ecologically significant until the Miocene, 6 to 7 million years ago. Remarkably, some charcoalified fossils preserve tissue organised into the Kranz anatomy, with intact bundle sheath cells, allowing the presence C4 metabolism to be identified. Isotopic markers are used to deduce their distribution and significance.
C3 plants preferentially use the lighter of two isotopes of carbon in the atmosphere, 12C, which is more readily involved in the chemical pathways involved in its fixation. Because C4 metabolism involves a further chemical step, this effect is accentuated. Plant material can be analysed to deduce the ratio of the heavier 13C to 12C. This ratio is denoted δ13C. C3 plants are on average around 14‰ (parts per thousand) lighter than the atmospheric ratio, while C4 plants are about 28‰ lighter. The δ13C of CAM plants depends on the percentage of carbon fixed at night relative to what is fixed in the day, being closer to C3 plants if they fix most carbon in the day and closer to C4 plants if they fix all their carbon at night.
Original fossil material in sufficient quantity to analyse the
grass itself is scarce, but horses provide a good proxy. They were
globally widespread in the period of interest, and browsed almost
exclusively on grasses. There's an old phrase in isotope paleontology,
"you are what you eat (plus a little bit)" – this refers to the fact
that organisms reflect the isotopic composition of whatever they eat,
plus a small adjustment factor. There is a good record of horse teeth
throughout the globe, and their δ13C record shows a sharp negative inflection around 6 to 7 million years ago, during the Messinian that is interpreted as resulting from the rise of C4 plants on a global scale.
When is C4 an advantage?
While C4 enhances the efficiency of RuBisCO, the concentration of carbon is highly energy intensive. This means that C4 plants only have an advantage over C3 organisms in certain conditions: namely, high temperatures and low rainfall. C4 plants also need high levels of sunlight to thrive. Models suggest that, without wildfires removing shade-casting trees and shrubs, there would be no space for C4 plants. But, wildfires have occurred for 400 million years – why did C4 take so long to arise, and then appear independently so many times? The Carboniferous (~300 million years ago) had notoriously high oxygen levels – almost enough to allow spontaneous combustion – and very low CO2, but there is no C4 isotopic signature to be found. And there doesn't seem to be a sudden trigger for the Miocene rise.
During the Miocene, the atmosphere and climate were relatively stable. If anything, CO2 increased gradually from 14 to 9 million years ago before settling down to concentrations similar to the Holocene. This suggests that it did not have a key role in invoking C4 evolution. Grasses themselves (the group which would give rise to the most occurrences of C4) had probably been around for 60 million years or more, so had had plenty of time to evolve C4,
which, in any case, is present in a diverse range of groups and thus
evolved independently. There is a strong signal of climate change in
South Asia;
increasing aridity – hence increasing fire frequency and intensity –
may have led to an increase in the importance of grasslands. However, this is difficult to reconcile with the North American record. It is possible that the signal is entirely biological, forced by the fire (and elephant?)
driven acceleration of grass evolution – which, both by increasing
weathering and incorporating more carbon into sediments, reduced
atmospheric CO2 levels. Finally, there is evidence that the onset of C4 from 9 to 7 million years ago
is a biased signal, which only holds true for North America, from where
most samples originate; emerging evidence suggests that grasslands
evolved to a dominant state at least 15Ma earlier in South America.
Evolution of transcriptional regulation
Transcription
factors and transcriptional regulatory networks play key roles in plant
development and stress responses, as well as their evolution. During
plant landing, many novel transcription factor families emerged and are
preferentially wired into the networks of multicellular development,
reproduction, and organ development, contributing to more complex
morphogenesis of land plants.
Evolution of secondary metabolism
Structure of Azadirachtin, a terpenoid produced by the Neem plant, which helps ward off microbes and insects. Many secondary metabolites have complex structures
Secondary metabolites are essentially low molecular weight compounds, sometimes having complex structures, that are not essential for the normal processes of growth, development, or reproduction. They function in processes as diverse as immunity, anti-herbivory, pollinator attraction, communication between plants, maintaining symbiotic associations with soil flora, or enhancing the rate of fertilization,
and hence are significant from the evo-devo perspective. Secondary
metabolites are structurally and functionally diverse, and it is
estimated that hundreds of thousands of enzymes might be involved in the
process of producing them, with about 15–25% of the genome coding for
these enzymes, and every species having its unique arsenal of secondary
metabolites. Many of these metabolites, such as salicylic acid are of medical significance to humans.
The purpose of producing so many secondary metabolites, with a significant proportion of the metabolome
devoted to this activity is unclear. It is postulated that most of
these chemicals help in generating immunity and, in consequence, the
diversity of these metabolites is a result of a constant arms race
between plants and their parasites. Some evidence supports this case. A
central question involves the reproductive cost to maintaining such a
large inventory of genes devoted to producing secondary metabolites.
Various models have been suggested that probe into this aspect of the
question, but a consensus on the extent of the cost has yet to be
established;
as it is still difficult to predict whether a plant with more secondary
metabolites increases its survival or reproductive success compared to
other plants in its vicinity.
Secondary metabolite production seems to have arisen quite early
during evolution. In plants, they seem to have spread out using
mechanisms including gene duplications or the evolution of novel genes.
Furthermore, research has shown that diversity in some of these
compounds may be positively selected for. Although the role of novel
gene evolution in the evolution of secondary metabolism is clear, there
are several examples where new metabolites have been formed by small
changes in the reaction. For example, cyanogen glycosides have been proposed to have evolved multiple times in different plant lineages. There are several such instances of convergent evolution. For example, enzymes for synthesis of limonene – a terpene –
are more similar between angiosperms and gymnosperms than to their own
terpene synthesis enzymes. This suggests independent evolution of the
limonene biosynthetic pathway in these two lineages.
Coevolution of plants and fungal parasites
An
additional contributing factor in some plants leading to evolutionary
change is the force due to coevolution with fungal parasites. In an
environment with a fungal parasite, which is common in nature, the
plants must make adaptation in an attempt to evade the harmful effects
of the parasite.
Whenever a parasitic fungus is siphoning limited resources away
from a plant, there is selective pressure for a phenotype that is better
able to prevent parasitic attack from fungi. At the same time, fungi
that are better equipped to evade the defenses of the plant will have
greater fitness level. The combination of these two factors leads to an
endless cycle of evolutionary change in the host-pathogen system.
Because each species in the relationship is influenced by a
constantly changing symbiont, evolutionary change usually occurs at a
faster pace than if the other species was not present. This is true of
most instances of coevolution. This makes the ability of a population to
quickly evolve vital to its survival. Also, if the pathogenic species
is too successful and threatens the survival and reproductive success of
the host plants, the pathogenic fungi risk losing their nutrient source
for future generations. These factors create a dynamic that shapes the
evolutionary changes in both species generation after generation.
Genes that code for defense mechanisms in plants must keep
changing to keep up with the parasite that constantly works to evade the
defenses. Genes that code for attachment mechanisms are the most
dynamic and are directly related to the evading ability of the fungi.
The greater the changes in these genes, the more change in the
attachment mechanism. After selective forces on the resulting
phenotypes, evolutionary change that promotes evasion of host defenses
occurs.
Fungi not only evolve to avoid the defenses of the plants, but
they also attempt to prevent the plant from enacting the mechanisms to
improve its defenses. Anything the fungi can do to slow the evolution
process of the host plants will improve the fitness of future
generations because the plant will not be able to keep up with the
evolutionary changes of the parasite. One of the main processes by which
plants quickly evolve in response to the environment is sexual
reproduction. Without sexual reproduction, advantageous traits could not
be spread through the plant population as quickly allowing the fungi to
gain a competitive advantage. For this reason, the sexual reproductive
organs of plants are targets for attacks by fungi. Studies have shown
that many different current types of obligate parasitic plant fungi have
developed mechanisms to disable or otherwise affect the sexual
reproduction of the plants. If successful, the sexual reproduction
process slows for the plant, thus slowing down evolutionary change or in
extreme cases, the fungi can render the plant sterile creating an
advantage for the pathogens. It is unknown exactly how this adaptive
trait developed in fungi, but it is clear that the relationship to the
plant forced the development of the process.
Some researchers are also studying how a range of factors affect
the rate of evolutionary change and the outcomes of change in different
environments. For example, as with most evolution, increases in
heritability in a population allow for a greater evolutionary response
in the presence of selective pressure. For traits specific to the
plant-fungi coevolution, researchers have studied how the virulence of
the invading pathogen affects the coevolution. Studies involving Mycosphaerella graminicola
have consistently showed that virulence of a pathogen does not have a
significant impact on the evolutionary track of the host plant.
There can be other factors in that can affect the process of
coevolution. For example, in small populations, selection is a
relatively weaker force on the population due to genetic drift.
Genetic drift increases the likelihood of having fixed alleles which
decreases the genetic variance in the population. Therefore, if there is
only a small population of plants in an area with the ability to
reproduce together, genetic drift may counteract the effects of
selection putting the plant in a disadvantageous position to fungi which
can evolve at a normal rate. The variance in both the host and pathogen
population is a major determinant of evolutionary success compared to
the other species. The greater the genetic variance, the faster the
species can evolve to counteract the other organism’s avoidance or
defensive mechanisms.
Due to the process of pollination for plants, the effective
population size is normally larger than for fungi because pollinators
can link isolated populations in a way that the fungus is not able. This
means positive traits that evolve in non-adjacent but close areas can
be passed to nearby areas. Fungi must individually evolve to evade host
defenses in each area. This is obviously a clear competitive advantage
for the host plants. Sexual reproduction with a broad, high variance
population leads to fast evolutionary change and higher reproductive
success of offspring.
Environment and climate patterns also play a role in evolutionary
outcomes. Studies with oak trees and an obligate fungal parasite at
different altitudes clearly show this distinction. For the same species,
different altitudinal positions had drastically different rates of
evolution and changes in the response to the pathogens due to the
organism also in a selective environment due to their surroundings.
Coevolution is a process that is related to the red queen hypothesis.
Both the host plant and parasitic fungi have to continue to survive to
stay in their ecological niche. If one of the two species in the
relationship evolves at a significantly faster rate than the other, the
slower species will be at a competitive disadvantage and risk the loss
of nutrients. Because the two species in the system are so closely
linked, they respond to external environment factors together and each
species affects the evolutionary outcome of the other. In other words,
each species exerts selective pressure on the other. Population size is
also a major factor in the outcome because differences in gene flow and
genetic drift could cause evolutionary changes that do not match the
direction of selection expected by forces due to the other organism.
Coevolution is an important phenomenon necessary for understanding the
vital relationship between plants and their fungal parasites.