Cankers caused by Chestnut blight, a disease that affects the chestnut tree
Plant disease resistance protects plants from pathogens
in two ways: by pre-formed structures and chemicals, and by
infection-induced responses of the immune system. Relative to a
susceptible plant, disease resistance is the reduction of pathogen growth on or in the plant (and hence a reduction of disease), while the term disease tolerance
describes plants that exhibit little disease damage despite substantial
pathogen levels. Disease outcome is determined by the three-way
interaction of the pathogen, the plant and the environmental conditions
(an interaction known as the disease triangle).
Defense-activating compounds can move cell-to-cell and
systematically through the plant's vascular system. However, plants do
not have circulating immune cells, so most cell types exhibit a broad suite of antimicrobial defenses. Although obvious qualitative
differences in disease resistance can be observed when multiple
specimens are compared (allowing classification as “resistant” or
“susceptible” after infection by the same pathogen strain at similar
inoculum levels in similar environments), a gradation of quantitative differences in disease resistance is more typically observed between plant strains or genotypes.
Plants consistently resist certain pathogens but succumb to others;
resistance is usually specific to certain pathogen species or pathogen
strains.
Background
Plant
disease resistance is crucial to the reliable production of food, and
it provides significant reductions in agricultural use of land, water,
fuel and other inputs. Plants in both natural and cultivated populations
carry inherent disease resistance, but this has not always protected
them.
The late blight Irish potato famine of the 1840s was caused by the oomycete Phytophthora infestans. The world’s first mass-cultivated banana cultivar Gros Michel was lost in the 1920s to Panama disease caused by the fungus Fusarium oxysporum. The current wheat stem rust, leaf rust and yellow stripe rust epidemics spreading from East Africa into the Indian subcontinent are caused by rust fungi Puccinia graminis and P. striiformis. Other epidemics include Chestnut blight, as well as recurrent severe plant diseases such as Rice blast, Soybean cyst nematode, Citrus canker.
Plant pathogens can spread rapidly over great distances, vectored
by water, wind, insects, and humans. Across large regions and many crop
species, it is estimated that diseases typically reduce plant yields by
10% every year in more developed nations or agricultural systems, but
yield loss to diseases often exceeds 20% in less developed settings.
However, disease control is reasonably successful for most crops.
Disease control is achieved by use of plants that have been bred for
good resistance to many diseases, and by plant cultivation approaches
such as crop rotation, pathogen-free seed, appropriate planting date and plant density, control of field moisture, and pesticide use.
Common disease resistance mechanisms
Pre-formed structures and compounds
secondary plant wall
- Plant cuticle/surface
- Plant cell walls
- Antimicrobial chemicals (for example: glucosides, saponins)
- Antimicrobial proteins
- Enzyme inhibitors
- Detoxifying enzymes that break down pathogen-derived toxins
- Receptors that perceive pathogen presence and activate inducible plant defences
Inducible post-infection plant defenses
- Cell wall reinforcement (cellulose, lignin, suberin, cell wall proteins)
- Antimicrobial chemicals, including reactive oxygen species such as hydrogen peroxide or peroxynitrite, or more complex phytoalexins such as genistein or camalexin
- Antimicrobial proteins such as defensins, thionins, or PR-1
- Antimicrobial enzymes such as chitinases, beta-glucanases, or peroxidases
- Hypersensitive response - a rapid host cell death response associated with defence mediated by "Resistance genes."(Bryant, Tracy 2008).
Immune system
The
plant immune system carries two interconnected tiers of receptors, one
most frequently sensing molecules outside the cell and the other most
frequently sensing molecules inside the cell. Both systems sense
the intruder and respond by activating antimicrobial defenses in the
infected cell and neighboring cells. In some cases, defense-activating
signals spread to the rest of the plant or even to neighboring plants.
The two systems detect different types of pathogen molecules and classes
of plant receptor proteins.
The first tier is primarily governed by pattern recognition receptors that are activated by recognition of evolutionarily conserved pathogen or microbial–associated molecular patterns
(PAMPs or MAMPs). Activation of PRRs leads to intracellular signaling,
transcriptional reprogramming, and biosynthesis of a complex output
response that limits colonization. The system is known as PAMP-Triggered
Immunity or as Pattern-Triggered Immunity (PTI).
The second tier, primarily governed by R gene
products, is often termed effector-triggered immunity (ETI). ETI is
typically activated by the presence of specific pathogen "effectors" and
then triggers strong antimicrobial responses.
In addition to PTI and ETI, plant defenses can be activated by
the sensing of damage-associated compounds (DAMP), such as portions of
the plant cell wall released during pathogenic infection.
Responses activated by PTI and ETI receptors include ion channel gating, oxidative burst, cellular redox changes, or protein kinase
cascades that directly activate cellular changes (such as cell wall
reinforcement or antimicrobial production), or activate changes in gene expression that then elevate other defensive responses.
Plant immune systems show some mechanistic similarities with the immune systems of insects and mammals, but also exhibit many plant-specific characteristics.
The two above-described tiers are central to plant immunity but do not
fully describe plant immune systems. In addition, many specific
examples of apparent PTI or ETI violate common PTI/ETI definitions,
suggesting a need for broadened definitions and/or paradigms.
Pattern-triggered immunity
PAMPs, conserved molecules that inhabit multiple pathogen genera,
are referred to as MAMPs by many researchers. The defenses induced by
MAMP perception are sufficient to repel most pathogens. However,
pathogen effector proteins (see below) are adapted to suppress basal
defenses such as PTI. Many receptors for MAMPs (and DAMPs) have been
discovered. MAMPs and DAMPs are often detected by transmembrane
receptor-kinases that carry LRR or LysM extracellular domains.
Effector triggered immunity
Effector Triggered Immunity (ETI) is activated by the presence of pathogen effectors. The ETI response is reliant on R genes, and is activated by specific pathogen strains. Plant ETI often causes an apoptotic hypersensitive response.
R genes and R proteins
Plants have evolved R genes
(resistance genes) whose products mediate resistance to specific virus,
bacteria, oomycete, fungus, nematode or insect strains. R gene products
are proteins that allow recognition of specific pathogen effectors,
either through direct binding or by recognition of the effector's
alteration of a host protein. Many R genes encode NB-LRR proteins (proteins with nucleotide-binding and leucine-rich repeat
domains, also known as NLR proteins or STAND proteins, among other
names). Most plant immune systems carry a repertoire of 100-600
different R gene homologs. Individual R genes have been demonstrated to
mediate resistance to specific virus, bacteria, oomycete, fungus,
nematode or insect strains. R gene products control a broad set of
disease resistance responses whose induction is often sufficient to stop
further pathogen growth/spread.
Studied R genes usually confer specificity for particular strains
of a pathogen species (those that express the recognized effector). As
first noted by Harold Flor in his mid-20th century formulation of the gene-for-gene relationship,
a plant R gene has specificity for a pathogen avirulence gene (Avr
gene). Avirulence genes are now known to encode effectors. The
pathogen Avr gene must have matched specificity with the R gene for that
R gene to confer resistance, suggesting a receptor/ligand interaction for Avr and R genes.
Alternatively, an effector can modify its host cellular target (or a
molecular decoy of that target), and the R gene product (NLR protein)
activates defenses when it detects the modified form of the host target
or decoy.
Effector biology
Effectors
are central to the pathogenic or symbiotic potential of microbes and
microscopic plant-colonizing animals such as nematodes.
Effectors typically are proteins that are delivered outside the microbe
and into the host cell. These colonist-derived effectors manipulate the
host's cell physiology and development. As such, effectors offer
examples of co-evolution (example: a fungal protein that functions
outside of the fungus but inside of plant cells has evolved to take on
plant-specific functions). Pathogen host range is determined, among
other things, by the presence of appropriate effectors that allow
colonization of a particular host.
Pathogen-derived effectors are a powerful tool to identify plant
functions that play key roles in disease and in disease resistance.
Apparently most effectors function to manipulate host physiology to
allow disease to occur. Well-studied bacterial plant pathogens typically
express a few dozen effectors, often delivered into the host by a Type III secretion apparatus. Fungal, oomycete and nematode plant pathogens apparently express a few hundred effectors.
So-called "core" effectors are defined operationally by their
wide distribution across the population of a particular pathogen and
their substantial contribution to pathogen virulence. Genomics can be
used to identify core effectors, which can then be used to discover new R
gene alleles, which can be used in plant breeding for disease resistance.
Small RNAs and RNA interference
Plant
sRNA pathways are understood to be important components of
pathogen-associated molecular pattern (PAMP)-triggered immunity (PTI)
and effector-triggered immunity (ETI).
Bacteria‐induced miRNAs in Arabidopsis have been shown to influence
hormonal signalling including auxin, abscisic acid (ABA), jasmonic acid
(JA) and salicylic acid (SA).
Advances in genome‐wide studies revealed a massive adaptation of host
miRNA expression patterns after infection by fungal pathogens Fusarium virguliforme, Erysiphe graminis, Verticillium dahliae, and Cronartium quercuum, and the oomycete Phytophthora sojae.
Changes to sRNA expression in response to fungal pathogens indicate
that gene silencing may be involved in this defense pathway. However,
there is also evidence that the antifungal defense response to Colletotrichum
spp. infection in maize is not entirely regulated by specific miRNA
induction, but may instead act to fine-tune the balance between genetic
and metabolic components upon infection.
Transport of sRNAs during infection is likely facilitated by extracellular vesicles (EVs) and multivesicular bodies (MVBs).
The composition of RNA in plant EVs has not been fully evaluated, but
it is likely that they are, in part, responsible for trafficking RNA.
Plants can transport viral RNAs, mRNAs, microRNAs (miRNAs) and small
interfering RNAs (siRNAs) systemically through the phloem.
This process is thought to occur through the plasmodesmata and involves
RNA-binding proteins that assist RNA localization in mesophyll cells.
Although they have been identified in the phloem with mRNA, there is no
determinate evidence that they mediate long-distant transport of RNAs.
EVs may therefore contribute to an alternate pathway of RNA loading
into the phloem, or could possibly transport RNA through the apoplast.
There is also evidence that plant EVs can allow for interspecies
transfer of sRNAs by RNA interference such as Host-Induced Gene
Silencing (HIGS). The transport of RNA between plants and fungi seems to be bidirectional as sRNAs from the fungal pathogen Botrytis cinerea have been shown to target host defense genes in Arabidopsis and tomato.
Species-level resistance
In
a small number of cases, plant genes are effective against an entire
pathogen species, even though that species that is pathogenic on other
genotypes of that host species. Examples include barley MLO against powdery mildew, wheat Lr34 against leaf rust and wheat Yr36 against wheat stripe rust.
An array of mechanisms for this type of resistance may exist depending
on the particular gene and plant-pathogen combination. Other reasons for
effective plant immunity can include a lack of coadaptation
(the pathogen and/or plant lack multiple mechanisms needed for
colonization and growth within that host species), or a particularly
effective suite of pre-formed defenses.
Signaling mechanisms
Perception of pathogen presence
Plant defense signaling is activated by the pathogen-detecting receptors that are described in an above section. The activated receptors frequently elicit reactive oxygen and nitric oxide production, calcium, potassium and proton ion fluxes, altered levels of salicylic acid and other hormones and activation of MAP kinases and other specific protein kinases. These events in turn typically lead to the modification of proteins that control gene transcription, and the activation of defense-associated gene expression.
Transcription factors and the hormone response
Numerous genes and/or proteins as well as other molecules have been identified that mediate plant defense signal transduction. Cytoskeleton and vesicle trafficking dynamics help to orient plant defense responses toward the point of pathogen attack.
Mechanisms of transcription factors and hormones
Plant immune system activity is regulated in part by signaling hormones such as:
There can be substantial cross-talk among these pathways.
Regulation by degradation
As
with many signal transduction pathways, plant gene expression during
immune responses can be regulated by degradation. This often occurs when
hormone binding to hormone receptors stimulates ubiquitin-associated
degradation of repressor proteins that block expression of certain
genes. The net result is hormone-activated gene expression. Examples:
- Auxin: binds to receptors that then recruit and degrade repressors of transcriptional activators that stimulate auxin-specific gene expression.
- Jasmonic acid: similar to auxin, except with jasmonate receptors impacting jasmonate-response signaling mediators such as JAZ proteins.
- Gibberellic acid: Gibberellin causes receptor conformational changes and binding and degradation of Della proteins.
- Ethylene: Inhibitory phosphorylation of the EIN2 ethylene response activator is blocked by ethylene binding. When this phosphorylation is reduced, EIN2 protein is cleaved and a portion of the protein moves to the nucleus to activate ethylene-response gene expression.
Ubiquitin and E3 signaling
Ubiquitination plays a central role in cell signaling that regulates processes including protein degradation and immunological response.
Although one of the main functions of ubiquitin is to target proteins
for destruction, it is also useful in signaling pathways, hormone
release, apoptosis and translocation of materials throughout the cell.
Ubiquitination is a component of several immune responses. Without
ubiquitin's proper functioning, the invasion of pathogens and other
harmful molecules would increase dramatically due to weakened immune
defenses.
E3 signaling
The E3 Ubiquitin ligase enzyme is a main component that provides specificity in protein degradation pathways, including immune signaling pathways. The E3 enzyme components can be grouped by which domains they contain and include several types. These include the Ring and U-box single subunit, HECT, and CRLs.
Plant signaling pathways including immune responses are controlled by
several feedback pathways, which often include negative feedback; and
they can be regulated by De-ubiquitination enzymes, degradation of
transcription factors and the degradation of negative regulators of
transcription.
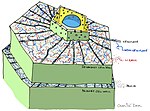
This
image depicts the pathways taken during responses in plant immunity. It
highlights the role and effect ubiquitin has in regulating the pathway.
Plant breeding for disease resistance
Plant
breeders emphasize selection and development of disease-resistant plant
lines. Plant diseases can also be partially controlled by use of pesticides and by cultivation practices such as crop rotation,
tillage, planting density, disease-free seeds and cleaning of
equipment, but plant varieties with inherent (genetically determined)
disease resistance are generally preferred.
Breeding for disease resistance began when plants were first
domesticated. Breeding efforts continue because pathogen populations are
under selection pressure
for increased virulence, new pathogens appear, evolving cultivation
practices and changing climate can reduce resistance and/or strengthen
pathogens, and plant breeding for other traits can disrupt prior
resistance. A plant line with acceptable resistance against one pathogen may lack resistance against others.
Breeding for resistance typically includes:
- Identification of plants that may be less desirable in other ways, but which carry a useful disease resistance trait, including wild plant lines that often express enhanced resistance.
- Crossing of a desirable but disease-susceptible variety to a plant that is a source of resistance.
- Growth of breeding candidates in a disease-conducive setting, possibly including pathogen inoculation. Attention must be paid to the specific pathogen isolates, to address variability within a single pathogen species.
- Selection of disease-resistant individuals that retain other desirable traits such as yield, quality and including other disease resistance traits.
Resistance is termed durable if it continues to be effective over multiple years of widespread use as pathogen populations evolve. "Vertical resistance" is specific to certain races or strains of a pathogen species, is often controlled by single R genes and can be less durable. Horizontal or broad-spectrum resistance
against an entire pathogen species is often only incompletely
effective, but more durable, and is often controlled by many genes that
segregate in breeding populations.
Crops such as potato, apple, banana and sugarcane are often propagated by vegetative reproduction
to preserve highly desirable plant varieties, because for these
species, outcrossing seriously disrupts the preferred traits. See also asexual propagation. Vegetatively propagated crops may be among the best targets for resistance improvement by the biotechnology method of plant transformation to manage genes that affect disease resistance.
Scientific breeding for disease resistance originated with Sir Rowland Biffen,
who identified a single recessive gene for resistance to wheat yellow
rust. Nearly every crop was then bred to include disease resistance (R)
genes, many by introgression from compatible wild relatives.
GM or transgenic engineered disease resistance
The term GM ("genetically modified") is often used as a synonym of transgenic
to refer to plants modified using recombinant DNA technologies. Plants
with transgenic/GM disease resistance against insect pests have been
extremely successful as commercial products, especially in maize and
cotton, and are planted annually on over 20 million hectares in over 20
countries worldwide. Transgenic plant disease resistance against microbial pathogens was first demonstrated in 1986. Expression of viral coat protein gene sequences conferred virus resistance via small RNAs. This proved to be a widely applicable mechanism for inhibiting viral replication. Combining coat protein genes from three different viruses, scientists developed squash
hybrids with field-validated, multiviral resistance. Similar levels of
resistance to this variety of viruses had not been achieved by
conventional breeding.
A similar strategy was deployed to combat papaya ringspot virus, which by 1994 threatened to destroy Hawaii’s papaya
industry. Field trials demonstrated excellent efficacy and high fruit
quality. By 1998 the first transgenic virus-resistant papaya was
approved for sale. Disease resistance has been durable for over 15
years. Transgenic papaya accounts for ~85% of Hawaiian production. The
fruit is approved for sale in the U.S., Canada and Japan.
Potato lines expressing viral replicase sequences that confer
resistance to potato leafroll virus were sold under the trade names
NewLeaf Y and NewLeaf Plus, and were widely accepted in commercial
production in 1999-2001, until McDonald's Corp. decided not to purchase
GM potatoes and Monsanto decided to close their NatureMark potato
business.
NewLeaf Y and NewLeaf Plus potatoes carried two GM traits, as they
also expressed Bt-mediated resistance to Colorado potato beetle.
No other crop with engineered disease resistance against
microbial pathogens had reached the market by 2013, although more than a
dozen were in some state of development and testing.
| Publication year | Crop | Disease resistance | Mechanism | Development status |
|---|---|---|---|---|
| 2012 | Tomato | Bacterial spot | R gene from pepper | 8 years of field trials |
| 2012 | Rice | Bacterial blight and bacterial streak | Engineered E gene | Laboratory |
| 2012 | Wheat | Powdery mildew | Overexpressed R gene from wheat | 2 years of field trials at time of publication |
| 2011 | Apple | Apple scab fungus | Thionin gene from barley | 4 years of field trials at time of publication |
| 2011 | Potato | Potato virus Y | Pathogen-derived resistance | 1 year of field trial at time of publication |
| 2010 | Apple | Fire blight | Antibacterial protein from moth | 12 years of field trials at time of publication |
| 2010 | Tomato | Multibacterial resistance | PRR from Arabidopsis | Laboratory scale |
| 2010 | Banana | Xanthomonas wilt | Novel gene from pepper | Now in field trial |
| 2009 | Potato | Late blight | R genes from wild relatives | 3 years of field trials |
| 2009 | Potato | Late blight | R gene from wild relative | 2 years of field trials at time of publication |
| 2008 | Potato | Late blight | R gene from wild relative | 2 years of field trials at time of publication |
| 2008 | Plum | Plum pox virus | Pathogen-derived resistance | Regulatory approvals, no commercial sales |
| 2005 | Rice | Bacterial streak | R gene from maize | Laboratory |
| 2002 | Barley | Stem rust | Resting lymphocyte kinase (RLK) gene from resistant barley cultivar | Laboratory |
| 1997 | Papaya | Ring spot virus | Pathogen-derived resistance | Approved and commercially sold since 1998, sold into Japan since 2012 |
| 1995 | Squash | Three mosaic viruses | Pathogen-derived resistance | Approved and commercially sold since 1994 |
| 1993 | Potato | Potato virus X | Mammalian interferon-induced enzyme | 3 years of field trials at time of publication |
PRR transfer
Research
aimed at engineered resistance follows multiple strategies. One is to
transfer useful PRRs into species that lack them. Identification of
functional PRRs and their transfer to a recipient species that lacks an
orthologous receptor could provide a general pathway to additional
broadened PRR repertoires. For example, the Arabidopsis PRR EF-Tu receptor (EFR) recognizes the bacterial translation elongation factor EF-Tu. Research performed at Sainsbury Laboratory demonstrated that deployment of EFR into either Nicotiana benthamianaor Solanum lycopersicum (tomato), which cannot recognize EF-Tu,
conferred resistance to a wide range of bacterial pathogens. EFR
expression in tomato was especially effective against the widespread and
devastating soil bacterium Ralstonia solanacearum. Conversely, the tomato PRR Verticillium 1 (Ve1) gene can be transferred from tomato to Arabidopsis, where it confers resistance to race 1 Verticillium isolates.
Stacking
The
second strategy attempts to deploy multiple NLR genes simultaneously, a
breeding strategy known as stacking. Cultivars generated by either
DNA-assisted molecular breeding or gene transfer
will likely display more durable resistance, because pathogens would
have to mutate multiple effector genes. DNA sequencing allows
researchers to functionally “mine” NLR genes from multiple
species/strains.
The avrBs2 effector gene from Xanthomona perforans
is the causal agent of bacterial spot disease of pepper and tomato. The
first “effector-rationalized” search for a potentially durable R gene
followed the finding that avrBs2 is found in most disease-causing Xanthomonas species and is required for pathogen fitness. The Bs2 NLR gene from the wild pepper, Capsicum chacoense,
was moved into tomato, where it inhibited pathogen growth. Field trials
demonstrated robust resistance without bactericidal chemicals. However,
rare strains of Xanthomonas overcame Bs2-mediated resistance in pepper by acquisition of avrBs2
mutations that avoid recognition but retain virulence. Stacking R genes
that each recognize a different core effector could delay or prevent
adaptation.
More than 50 loci in wheat strains confer disease resistance
against wheat stem, leaf and yellow stripe rust pathogens. The Stem rust
35 (Sr35) NLR gene, cloned from a diploid relative of cultivated wheat, Triticum monococcum, provides resistance to wheat rust isolate Ug99. Similarly, Sr33, from the wheat relative Aegilops tauschii, encodes a wheat ortholog to barley Mla powdery mildew–resistance genes. Both genes are unusual in wheat and its relatives. Combined with the Sr2 gene that acts additively with at least Sr33, they could provide durable disease resistance to Ug99 and its derivatives.
Executor genes
Another
class of plant disease resistance genes opens a “trap door” that
quickly kills invaded cells, stopping pathogen proliferation.
Xanthomonas and Ralstonia transcription activator–like
(TAL) effectors are DNA-binding proteins that activate host gene
expression to enhance pathogen virulence. Both the rice and pepper
lineages independently evolved TAL-effector binding sites that instead
act as an executioner that induces hypersensitive host cell death when
up-regulated. Xa27 from rice and Bs3 and Bs4c from pepper, are
such “executor” (or "executioner") genes that encode non-homologous
plant proteins of unknown function. Executor genes are expressed only in
the presence of a specific TAL effector.
Engineered executor genes were demonstrated by successfully redesigning the pepper Bs3
promoter to contain two additional binding sites for TAL effectors from
disparate pathogen strains. Subsequently, an engineered executor gene
was deployed in rice by adding five TAL effector binding sites to the Xa27 promoter. The synthetic Xa27 construct conferred resistance against Xanthomonas bacterial blight and bacterial leaf streak species.
Host susceptibility alleles
Most
plant pathogens reprogram host gene expression patterns to directly
benefit the pathogen. Reprogrammed genes required for pathogen survival
and proliferation can be thought of as “disease-susceptibility genes.”
Recessive resistance genes are disease-susceptibility candidates. For
example, a mutation disabled an Arabidopsis gene encoding pectate lyase (involved in cell wall degradation), conferring resistance to the powdery mildew pathogen Golovinomyces cichoracearum. Similarly, the Barley MLO gene and spontaneously mutated pea and tomato MLO orthologs also confer powdery mildew resistance.
Lr34 is a gene that provides partial resistance to leaf and yellow rusts and powdery mildew in wheat. Lr34 encodes an adenosine triphosphate
(ATP)–binding cassette (ABC) transporter. The dominant allele that
provides disease resistance was recently found in cultivated wheat (not
in wild strains) and, like MLO provides broad-spectrum resistance in barley.
Natural alleles of host translation elongation initiation factors eif4e and eif4g are also recessive viral-resistance genes. Some have been deployed to control potyviruses
in barley, rice, tomato, pepper, pea, lettuce and melon. The discovery
prompted a successful mutant screen for chemically induced eif4e alleles in tomato.
Natural promoter variation can lead to the evolution of recessive
disease-resistance alleles. For example, the recessive resistance gene xa13 in rice is an allele of Os-8N3. Os-8N3 is transcriptionally activated byXanthomonas oryzae pv. oryzae strains that express the TAL effector PthXo1. The xa13 gene has a mutated effector-binding element in its promoter that eliminates PthXo1 binding and renders these lines resistant to strains that rely on PthXo1. This finding also demonstrated that Os-8N3 is required for susceptibility.
Xa13/Os-8N3 is required for pollen development, showing that such
mutant alleles can be problematic should the disease-susceptibility
phenotype alter function in other processes. However, mutations in the Os11N3 (OsSWEET14) TAL effector–binding element were made by fusing TAL effectors to nucleases (TALENs). Genome-edited rice plants with altered Os11N3 binding sites remained resistant to Xanthomonas oryzae pv. oryzae, but still provided normal development function.
Gene silencing
RNA silencing-based
resistance is a powerful tool for engineering resistant crops. The
advantage of RNAi as a novel gene therapy against fungal, viral and
bacterial infection in plants lies in the fact that it regulates gene
expression via messenger RNA degradation, translation repression and chromatin
remodelling through small non-coding RNAs. Mechanistically, the
silencing processes are guided by processing products of the
double-stranded RNA (dsRNA) trigger, which are known as small interfering RNAs and microRNAs.
Host range
Among the thousands of species of plant pathogenic microorganisms,
only a small minority have the capacity to infect a broad range of plant
species. Most pathogens instead exhibit a high degree of
host-specificity. Non-host plant species are often said to express non-host resistance. The term host resistance
is used when a pathogen species can be pathogenic on the host species
but certain strains of that plant species resist certain strains of the
pathogen species. The causes of host resistance and non-host resistance
can overlap. Pathogen host range is determined, among other things, by
the presence of appropriate effectors that allow colonization of a
particular host.
Pathogen host range can change quite suddenly if, for example, the
pathogen's capacity to synthesize a host-specific toxin or effector is
gained by gene shuffling/mutation, or by horizontal gene transfer.
Epidemics and population biology
Native
populations are often characterized by substantial genotype diversity
and dispersed populations (growth in a mixture with many other plant
species). They also have undergone of plant-pathogen coevolution.
Hence as long as novel pathogens are not introduced/do not evolve, such
populations generally exhibit only a low incidence of severe disease epidemics.
Monocrop agricultural systems provide an ideal environment for
pathogen evolution, because they offer a high density of target
specimens with similar/identical genotypes. The rise in mobility stemming from modern transportation systems provides pathogens with access to more potential targets.
Climate change can alter the viable geographic range of pathogen
species and cause some diseases to become a problem in areas where the
disease was previously less important.
These factors make modern agriculture more prone to disease
epidemics. Common solutions include constant breeding for disease
resistance, use of pesticides, use of border inspections and plant
import restrictions, maintenance of significant genetic diversity within
the crop gene pool,
and constant surveillance to accelerate initiation of appropriate
responses. Some pathogen species have much greater capacity to overcome
plant disease resistance than others, often because of their ability to
evolve rapidly and to disperse broadly.