Emission spectrum of a metal halide lamp.
A demonstration of the 589 nm D2 (left) and 590 nm D1 (right) emission sodium D lines using a wick with salt water in a flame
The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted due to an atom or molecule making a transition from a high energy state to a lower energy state. The photon energy of the emitted photon
is equal to the energy difference between the two states. There are
many possible electron transitions for each atom, and each transition
has a specific energy difference. This collection of different
transitions, leading to different radiated wavelengths, make up an emission spectrum. Each element's emission spectrum is unique. Therefore, spectroscopy
can be used to identify the elements in matter of unknown composition.
Similarly, the emission spectra of molecules can be used in chemical
analysis of substances.
Emission
In physics,
emission is the process by which a higher energy quantum mechanical
state of a particle becomes converted to a lower one through the
emission of a photon, resulting in the production of light.
The frequency of light emitted is a function of the energy of the
transition. Since energy must be conserved, the energy difference
between the two states equals the energy carried off by the photon. The
energy states of the transitions can lead to emissions over a very large
range of frequencies. For example, visible light is emitted by the coupling of electronic states in atoms and molecules (then the phenomenon is called fluorescence or phosphorescence). On the other hand, nuclear shell transitions can emit high energy gamma rays, while nuclear spin transitions emit low energy radio waves.
The emittance of an object quantifies how much light is emitted by it. This may be related to other properties of the object through the Stefan–Boltzmann law.
For most substances, the amount of emission varies with the temperature and the spectroscopic composition of the object, leading to the appearance of color temperature and emission lines. Precise measurements at many wavelengths allow the identification of a substance via emission spectroscopy.
Emission of radiation is typically described using semi-classical
quantum mechanics: the particle's energy levels and spacings are
determined from quantum mechanics,
and light is treated as an oscillating electric field that can drive a
transition if it is in resonance with the system's natural frequency.
The quantum mechanics problem is treated using time-dependent perturbation theory and leads to the general result known as Fermi's golden rule. The description has been superseded by quantum electrodynamics, although the semi-classical version continues to be more useful in most practical computations.
Origins
When the electrons in the atom are excited, for example by being heated, the additional energy
pushes the electrons to higher energy orbitals. When the electrons fall
back down and leave the excited state, energy is re-emitted in the form
of a photon.
The wavelength (or equivalently, frequency) of the photon is determined
by the difference in energy between the two states. These emitted
photons form the element's spectrum.
The fact that only certain colors appear in an element's atomic
emission spectrum means that only certain frequencies of light are
emitted. Each of these frequencies are related to energy by the formula:
- ,
where is the energy of the photon, is its frequency, and is Planck's constant.
This concludes that only photons with specific energies are emitted by the atom. The principle of the atomic emission spectrum explains the varied colors in neon signs, as well as chemical flame test results (described below).
The frequencies of light that an atom can emit are dependent on
states the electrons can be in. When excited, an electron moves to a
higher energy level or orbital. When the electron falls back to its
ground level the light is emitted.
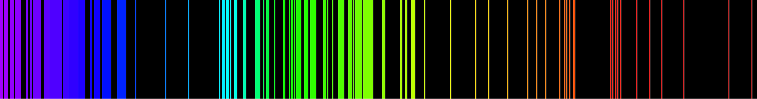
Emission spectrum of hydrogen
The above picture shows the visible light emission spectrum for hydrogen.
If only a single atom of hydrogen were present, then only a single
wavelength would be observed at a given instant. Several of the possible
emissions are observed because the sample contains many hydrogen atoms
that are in different initial energy states and reach different final
energy states. These different combinations lead to simultaneous
emissions at different wavelengths.
Emission spectrum of iron
Radiation from molecules
As well as the electronic transitions discussed above, the energy of a molecule can also change via rotational, vibrational, and vibronic
(combined vibrational and electronic) transitions. These energy
transitions often lead to closely spaced groups of many different
spectral lines, known as spectral bands. Unresolved band spectra may appear as a spectral continuum.
Emission spectroscopy
Light
consists of electromagnetic radiation of different wavelengths.
Therefore, when the elements or their compounds are heated either on a
flame or by an electric arc they emit energy in the form of light.
Analysis of this light, with the help of a spectroscope
gives us a discontinuous spectrum. A spectroscope or a spectrometer is
an instrument which is used for separating the components of light,
which have different wavelengths. The spectrum appears in a series of
lines called the line spectrum. This line spectrum is called an atomic
spectrum when it originates from an atom in elemental form. Each element
has a different atomic spectrum. The production of line spectra by the
atoms of an element indicate that an atom can radiate only a certain
amount of energy. This leads to the conclusion that bound electrons
cannot have just any amount of energy but only a certain amount of
energy.
The emission spectrum can be used to determine the composition of a material, since it is different for each element of the periodic table. One example is astronomical spectroscopy: identifying the composition of stars
by analysing the received light.
The emission spectrum characteristics of some elements are plainly
visible to the naked eye when these elements are heated. For example,
when platinum wire is dipped into a strontium nitrate solution and then inserted into a flame, the strontium atoms emit a red color. Similarly, when copper
is inserted into a flame, the flame becomes green. These definite
characteristics allow elements to be identified by their atomic emission
spectrum. Not all emitted lights are perceptible to the naked eye, as
the spectrum also includes ultraviolet rays and infrared lighting.
An emission is formed when an excited gas is viewed directly through a
spectroscope.
Schematic diagram of spontaneous emission
Emission spectroscopy is a spectroscopic technique which examines the wavelengths of photons emitted by atoms or molecules during their transition from an excited state to a lower energy state. Each element emits a characteristic set of discrete wavelengths according to its electronic structure,
and by observing these wavelengths the elemental composition of the
sample can be determined. Emission spectroscopy developed in the late
19th century and efforts in theoretical explanation of atomic emission
spectra eventually led to quantum mechanics.
There are many ways in which atoms can be brought to an excited state. Interaction with electromagnetic radiation is used in fluorescence spectroscopy, protons or other heavier particles in Particle-Induced X-ray Emission and electrons or X-ray photons in Energy-dispersive X-ray spectroscopy or X-ray fluorescence.
The simplest method is to heat the sample to a high temperature, after
which the excitations are produced by collisions between the sample
atoms. This method is used in flame emission spectroscopy, and it was also the method used by Anders Jonas Ångström when he discovered the phenomenon of discrete emission lines in the 1850s.
Although the emission lines are caused by a transition between
quantized energy states and may at first look very sharp, they do have a
finite width, i.e. they are composed of more than one wavelength of
light. This spectral line broadening has many different causes.
Emission spectroscopy is often referred to as optical emission spectroscopy because of the light nature of what is being emitted.
History
Emission lines from hot gases were first discovered by Ångström, and the technique was further developed by David Alter, Gustav Kirchhoff and Robert Bunsen.
Experimental technique in flame emission spectroscopy
The
solution containing the relevant substance to be analysed is drawn into
the burner and dispersed into the flame as a fine spray. The solvent
evaporates first, leaving finely divided solid particles which move to the hottest region of the flame where gaseous atoms and ions are produced. Here electrons are excited as described above. It is common for a monochromator to be used to allow for easy detection.
On a simple level, flame emission spectroscopy can be observed using just a flame and samples of metal salts. This method of qualitative analysis is called a flame test. For example, sodium salts placed in the flame will glow yellow from sodium ions, while strontium (used in road flares) ions color it red. Copper wire will create a blue colored flame, however in the presence of chloride gives green (molecular contribution by CuCl).
Emission coefficient
Emission coefficient is a coefficient in the power output per unit time of an electromagnetic source, a calculated value in physics. The emission coefficient of a gas varies with the wavelength of the light. It has units of ms−3sr−1. It is also used as a measure of environmental emissions (by mass) per MWh of electricity generated.
Scattering of light
In Thomson scattering
a charged particle emits radiation under incident light. The particle
may be an ordinary atomic electron, so emission coefficients have
practical applications.
If X dV dΩ dλ is the energy scattered by a volume element dV into solid angle dΩ between wavelengths λ and λ+dλ per unit time then the Emission coefficient is X.
The values of X in Thomson scattering can be predicted from incident flux, the density of the charged particles and their Thomson differential cross section (area/solid angle).
Spontaneous emission
A warm body emitting photons has a monochromatic emission coefficient relating to its temperature and total power radiation. This is sometimes called the second Einstein coefficient, and can be deduced from quantum mechanical theory.