Example
of bent electron arrangement. Shows location of unpaired electrons,
bonded atoms, and bond angles. (Water molecule) The bond angle for
water is 104.5°.
Valence shell electron pair repulsion (VSEPR) theory is a model used in chemistry to predict the geometry of individual molecules from the number of electron pairs surrounding their central atoms. It is also named the Gillespie-Nyholm theory after its two main developers, Ronald Gillespie and Ronald Nyholm. The acronym "VSEPR" is pronounced either "ves-pur" or "vuh-seh-per".
The premise of VSEPR is that the valence electron
pairs surrounding an atom tend to repel each other and will, therefore,
adopt an arrangement that minimizes this repulsion, thus determining
the molecule's geometry. Gillespie has emphasized that the electron-electron repulsion due to the Pauli exclusion principle is more important in determining molecular geometry than the electrostatic repulsion.
VSEPR theory is based on observable electron density rather than mathematical wave functions and hence unrelated to orbital hybridisation,
although both address molecular shape. While it is mainly qualitative,
VSEPR has a quantitative basis in quantum chemical topology (QCT)
methods such as the electron localization function (ELF) and the quantum theory of atoms in molecules (QTAIM).
History
The
idea of a correlation between molecular geometry and number of valence
electron pairs (both shared and unshared pairs) was originally proposed
in 1939 by Ryutaro Tsuchida in Japan, and was independently presented in a Bakerian Lecture in 1940 by Nevil Sidgwick and Herbert Powell of the University of Oxford. In 1957, Ronald Gillespie and Ronald Sydney Nyholm of University College London refined this concept into a more detailed theory, capable of choosing between various alternative geometries.
In recent years, VSEPR theory has been criticized as an outdated
model from the standpoint of both scientific accuracy and pedagogical
value.
In particular, the equivalent lone pairs of water and carbonyl
compounds in VSEPR theory neglect fundamental differences in the
symmetries (σ vs. π) of molecular orbitals and natural bond orbitals
that correspond to them, a difference that is sometimes chemically
significant. Furthermore, there is little evidence, computational or
experimental, proposing that lone pairs are "bigger" than bonding pairs.
It has been suggested that Bent's rule
is capable of replacing VSEPR as a simple model for explaining
molecular structure. Nevertheless, VSEPR theory captures many of the
essential features of the structure and electron distribution of simple
molecules, and most undergraduate general chemistry courses continue to teach it.
Overview
VSEPR
theory is used to predict the arrangement of electron pairs around
non-hydrogen atoms in molecules, especially simple and symmetric
molecules, where these key, central atoms participate in bonding to two
or more other atoms; the geometry of these key atoms and their
non-bonding electron pairs in turn determine the geometry of the larger
whole.
The number of electron pairs in the valence shell of a central atom is determined after drawing the Lewis structure of the molecule, and expanding it to show all bonding groups and lone pairs of electrons. In VSEPR theory, a double bond or triple bond are treated as a single bonding group. The sum of the number of atoms bonded to a central atom and the number of lone pairs formed by its nonbonding valence electrons is known as the central atom's steric number.
The electron pairs (or groups if multiple bonds are present) are
assumed to lie on the surface of a sphere centered on the central atom
and tend to occupy positions that minimize their mutual repulsions by
maximizing the distance between them.
The number of electron pairs (or groups), therefore, determines the
overall geometry that they will adopt. For example, when there are two
electron pairs surrounding the central atom, their mutual repulsion is
minimal when they lie at opposite poles of the sphere. Therefore, the
central atom is predicted to adopt a linear geometry. If there
are 3 electron pairs surrounding the central atom, their repulsion is
minimized by placing them at the vertices of an equilateral triangle
centered on the atom. Therefore, the predicted geometry is trigonal. Likewise, for 4 electron pairs, the optimal arrangement is tetrahedral.
Degree of repulsion
The overall geometry is further refined by distinguishing between bonding and nonbonding electron pairs. The bonding electron pair shared in a sigma bond
with an adjacent atom lies further from the central atom than a
nonbonding (lone) pair of that atom, which is held close to its
positively charged nucleus. VSEPR theory therefore views repulsion by
the lone pair to be greater than the repulsion by a bonding pair. As
such, when a molecule has 2 interactions with different degrees of
repulsion, VSEPR theory predicts the structure where lone pairs occupy
positions that allow them to experience less repulsion. Lone pair–lone
pair (lp–lp) repulsions are considered stronger than lone pair–bonding
pair (lp–bp) repulsions, which in turn are considered stronger than
bonding pair–bonding pair (bp–bp) repulsions, distinctions that then
guide decisions about overall geometry when 2 or more non-equivalent
positions are possible. For instance, when 5 valence electron pairs surround a central atom, they adopt a trigonal bipyramidal molecular geometry with two collinear axial positions and three equatorial
positions. An electron pair in an axial position has three close
equatorial neighbors only 90° away and a fourth much farther at 180°,
while an equatorial electron pair has only two adjacent pairs at 90° and
two at 120°. The repulsion from the close neighbors at 90° is more
important, so that the axial positions experience more repulsion than
the equatorial positions; hence, when there are lone pairs, they tend to
occupy equatorial positions as shown in the diagrams of the next
section for steric number five.
The difference between lone pairs and bonding pairs may also be
used to rationalize deviations from idealized geometries. For example,
the H2O molecule has four electron pairs in its valence
shell: two lone pairs and two bond pairs. The four electron pairs are
spread so as to point roughly towards the apices of a tetrahedron.
However, the bond angle between the two O–H bonds is only 104.5°, rather
than the 109.5° of a regular tetrahedron, because the two lone pairs
(whose density or probability envelopes lie closer to the oxygen
nucleus) exert a greater mutual repulsion than the two bond pairs.
An advanced-level explanation replaces the above distinction with two rules:
- Bent's rule: An electron pair of a more electropositive ligand constitutes greater repulsion. This explains why the Cl in PClF4 prefers the equatorial position and why the bond angle in oxygen difluoride (103.8°) is smaller than that of water (104.5°). Lone pairs are then considered to be a special case of this rule, held by a "ghost ligand" in the limit of electropositivity.
- A higher bond order constitutes greater repulsion. This explains why in phosgene, the oxygen–chlorine bond angle (124.1°) is larger than the chlorine–chlorine bond angle (111.8°) even though chlorine is more electropositive than oxygen. In the carbonate ion, all three bond angles are equivalent due to resonance.
AXE method
The
"AXE method" of electron counting is commonly used when applying the
VSEPR theory. The electron pairs around a central atom are represented
by a formula AXnEm, where A represents the central atom and always has an implied subscript one. Each X represents a ligand (an atom bonded to A). Each E represents a lone pair of electrons on the central atom. The total number of X and E is known as the steric number. For example in a molecule AX3E2, the atom A has a steric number of 5.
Based on the steric number and distribution of Xs and Es,
VSEPR theory makes the predictions in the following tables. Note that
the geometries are named according to the atomic positions only and not
the electron arrangement. For example, the description of AX2E1 as a bent molecule means that the three atoms AX2 are not in one straight line, although the lone pair helps to determine the geometry.
| Molecule type |
Shape | Electron arrangement including lone pairs, shown in pale yellow | Geometry excluding lone pairs | Examples |
|---|---|---|---|---|
| AX2E0 | Linear | BeCl2, HgCl2, CO2 | ||
| AX2E1 | Bent | 
|

|
NO− 2, SO2, O3, CCl2 |
| AX2E2 | Bent | 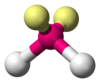
|

|
H2O, OF2 |
| AX2E3 | Linear | 
|
XeF2, XeCl2 | |
| AX3E0 | Trigonal planar | 
|

|
BF3, CO2− 3, NO− 3, SO3 |
| AX3E1 | Trigonal pyramidal | 
|

|
NH3, PCl3 |
| AX3E2 | T-shaped | 
|

|
ClF3, BrF3 |
| AX4E0 | Tetrahedral | 
|

|
CH4, PO3− 4, SO2− 4, ClO− 4, XeO4 |
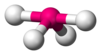
| AX4E1 | Seesaw or disphenoidal | 
|
|
SF4 |
| AX4E2 | Square planar | 
|

|
XeF4 |
| AX5E0 | Trigonal bipyramidal | 
|

|
PCl5 |
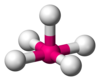
| AX5E1 | Square pyramidal | 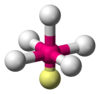
|
|
ClF5, BrF5, XeOF4 |
| AX5E2 | Pentagonal planar | 
|

|
XeF− 5 |
| AX6E0 | Octahedral | 
|
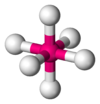
|
SF6, WCl6 |
| AX6E1 | Pentagonal pyramidal | 
|

|
XeOF− 5, IOF2− 5 |
| AX7E0 | Pentagonal bipyramidal | 
|

|
IF7 |
| AX8E0 | Square antiprismatic[11] | 
|

|
IF− 8, ZrF4− 8, ReF− 8 |
| AX9E0 | Tricapped trigonal prismatic | 
|

|
ReH2− 9 |
When the substituent
(X) atoms are not all the same, the geometry is still approximately
valid, but the bond angles may be slightly different from the ones where
all the outside atoms are the same. For example, the double-bond
carbons in alkenes like C2H4 are AX3E0, but the bond angles are not all exactly 120°. Likewise, SOCl2 is AX3E1, but because the X substituents are not identical, the X–A–X angles are not all equal.
As a tool in predicting the geometry adopted with a given number
of electron pairs, an often used physical demonstration of the principle
of minimal electron pair repulsion utilizes inflated balloons. Through
handling, balloons acquire a slight surface electrostatic charge that
results in the adoption of roughly the same geometries when they are
tied together at their stems as the corresponding number of electron
pairs. For example, five balloons tied together adopt the trigonal
bipyramidal geometry, just as do the five bonding pairs of a PCl5 molecule (AX5) or the two bonding and three non-bonding pairs of a XeF2 molecule (AX2E3). The molecular geometry of the former is also trigonal bipyramidal, whereas that of the latter is linear.
Possible geometries for steric numbers of 10, 11, 12, or 14 are bicapped square antiprismatic (or bicapped dodecadeltahedral), octadecahedral, icosahedral, and bicapped hexagonal antiprismatic, respectively. No compounds with steric numbers this high involving monodentate
ligands exist, and those involving multidentate ligands can often be
analysed more simply as complexes with lower steric numbers when some
multidentate ligands are treated as a unit.
Examples
The methane molecule (CH4) is tetrahedral because there are four pairs of electrons. The four hydrogen atoms are positioned at the vertices of a tetrahedron, and the bond angle is cos−1(−1⁄3) ≈ 109° 28′. This is referred to as an AX4 type of molecule. As mentioned above, A represents the central atom and X represents an outer atom.
The ammonia molecule (NH3) has three pairs of electrons involved in bonding, but there is a lone pair of electrons on the nitrogen atom.
It is not bonded with another atom; however, it influences the overall
shape through repulsions. As in methane above, there are four regions of
electron density. Therefore, the overall orientation of the regions of
electron density is tetrahedral. On the other hand, there are only three
outer atoms. This is referred to as an AX3E type molecule because the lone pair is represented by an E.
By definition, the molecular shape or geometry describes the geometric
arrangement of the atomic nuclei only, which is trigonal-pyramidal for
NH3.
Steric numbers of 7 or greater are possible, but are less common. The steric number of 7 occurs in iodine heptafluoride (IF7); the base geometry for a steric number of 7 is pentagonal bipyramidal. The most common geometry for a steric number of 8 is a square antiprismatic geometry. Examples of this include the octacyanomolybdate (Mo(CN)4−
8) and octafluorozirconate (ZrF4−
8) anions. The nonahydridorhenate ion (ReH2−
9) in potassium nonahydridorhenate is a rare example of a compound with a steric number of 9, which has a tricapped trigonal prismatic geometry.
8) and octafluorozirconate (ZrF4−
8) anions. The nonahydridorhenate ion (ReH2−
9) in potassium nonahydridorhenate is a rare example of a compound with a steric number of 9, which has a tricapped trigonal prismatic geometry.
Exceptions
There are groups of compounds where VSEPR fails to predict the correct geometry.
Some AX2E0 molecules
The gas phase structures of the triatomic halides of the heavier members of group 2, (i.e., calcium, strontium and barium halides, MX2), are not linear as predicted but are bent, (approximate X–M–X angles: CaF2, 145°; SrF2, 120°; BaF2, 108°; SrCl2, 130°; BaCl2, 115°; BaBr2, 115°; BaI2, 105°). It has been proposed by Gillespie
that this is caused by interaction of the ligands with the electron
core of the metal atom, polarising it so that the inner shell is not
spherically symmetric, thus influencing the molecular geometry.
Ab initio calculations have been cited to propose that contributions
from d orbitals in the shell below the valence shell are responsible,
together with the overlap of other orbitals- Disilynes are also bent, despite having no lone pairs.
Some AX2E2 molecules
One example of the AX2E2 geometry is molecular lithium oxide, Li2O,
a linear rather than bent structure, which is ascribed to its bonds
being essentially ionic and the strong lithium-lithium repulsion that
results. Another example is O(SiH3)2 with an Si–O–Si angle of 144.1°, which compares to the angles in Cl2O (110.9°), (CH3)2O (111.7°), and N(CH3)3 (110.9°).
Gillespie and Robinson rationalize the Si–O–Si bond angle based on the
observed ability of a ligand's lone pair to most greatly repel other
electron pairs when the ligand electronegativity is greater than or
equal to that of the central atom. In O(SiH3)2,
the central atom is more electronegative, and the lone pairs are less
localized and more weakly repulsive. The larger Si–O–Si bond angle
results from this and strong ligand-ligand repulsion by the relatively
large -SiH3 ligand. Burford et al showed through X-ray diffraction studies that Cl3Al–O–PCl3 has a linear Al–O–P bond angle and is therefore a non-VSEPR molecule.
Some AX6E1 and AX8E1 molecules
Xenon hexafluoride, which has a distorted octahedral geometry.
Some AX6E1 molecules, e.g. xenon hexafluoride (XeF6) and the Te(IV) and Bi(III) anions, TeCl2−
6, TeBr2−
6, BiCl3−
6, BiBr3−
6 and BiI3−
6, are octahedra, rather than pentagonal pyramids, and the lone pair does not affect the geometry to the degree predicted by VSEPR. Similarly, the octafluoroxenate ion (XeF2−
8) in nitrosonium octafluoroxenate(VI) is a square antiprism and not a bicapped trigonal prism (as predicted by VSEPR theory for an AX8E1 molecule), despite having a lone pair. One rationalization is that steric crowding of the ligands allows little or no room for the non-bonding lone pair; another rationalization is the inert pair effect.
6, TeBr2−
6, BiCl3−
6, BiBr3−
6 and BiI3−
6, are octahedra, rather than pentagonal pyramids, and the lone pair does not affect the geometry to the degree predicted by VSEPR. Similarly, the octafluoroxenate ion (XeF2−
8) in nitrosonium octafluoroxenate(VI) is a square antiprism and not a bicapped trigonal prism (as predicted by VSEPR theory for an AX8E1 molecule), despite having a lone pair. One rationalization is that steric crowding of the ligands allows little or no room for the non-bonding lone pair; another rationalization is the inert pair effect.
Transition metal molecules
Hexamethyltungsten, a transition metal compound whose geometry is different from main group coordination.
Many transition metal compounds have unusual geometries, which can be
ascribed to ligand bonding interaction with the d subshell and to
absence of valence shell lone pairs. Gillespie suggested that this interaction can be weak or strong. Weak interaction is dealt with by the Kepert model, while strong interaction produces bonding pairs that also occupy the respective antipodal points of the sphere. This is similar to predictions based on sd hybrid orbitals using the VALBOND theory. The repulsion of these bidirectional bonding pairs leads to a different prediction of shapes.
| Molecule type | Shape | Geometry | Examples |
|---|---|---|---|
| AX2 | Bent | 
|
VO+ 2 |
| AX3 | Trigonal pyramidal | 
|
CrO3 |
| AX4 | Tetrahedral | 
|
TiCl4 |
| AX5 | Square pyramidal | 
|
Ta(CH3)5 |
| AX6 | C3v Trigonal prismatic | 
|
W(CH3)6 |
The Kepert model cannot explain the formation of square planar complexes.
Odd-electron molecules
The
VSEPR theory can be extended to molecules with an odd number of
electrons by treating the unpaired electron as a "half electron pair" —
for example, Gillespie and Nyholm suggested that the decrease in the bond angle in the series NO+
2 (180°), NO2 (134°), NO−
2 (115°) indicates that a given set of bonding electron pairs exert a weaker repulsion on a single non-bonding electron than on a pair of non-bonding electrons. In effect, they considered nitrogen dioxide as an AX2E0.5 molecule, with a geometry intermediate between NO+
2 and NO−
2. Similarly, chlorine dioxide (ClO2) is an AX2E1.5 molecule, with a geometry intermediate between ClO+
2 and ClO−
2.
2 (180°), NO2 (134°), NO−
2 (115°) indicates that a given set of bonding electron pairs exert a weaker repulsion on a single non-bonding electron than on a pair of non-bonding electrons. In effect, they considered nitrogen dioxide as an AX2E0.5 molecule, with a geometry intermediate between NO+
2 and NO−
2. Similarly, chlorine dioxide (ClO2) is an AX2E1.5 molecule, with a geometry intermediate between ClO+
2 and ClO−
2.
Finally, the methyl radical (CH3) is predicted to be trigonal pyramidal like the methyl anion (CH−
3), but with a larger bond angle (as in the trigonal planar methyl cation (CH+
3)). However, in this case, the VSEPR prediction is not quite true, as CH3 is actually planar, although its distortion to a pyramidal geometry requires very little energy.
3), but with a larger bond angle (as in the trigonal planar methyl cation (CH+
3)). However, in this case, the VSEPR prediction is not quite true, as CH3 is actually planar, although its distortion to a pyramidal geometry requires very little energy.



