A replica of one of Geiger and Marsden's apparatus
The Geiger–Marsden experiments (also called the Rutherford gold foil experiment) were a landmark series of experiments by which scientists discovered that every atom contains a nucleus where all of its positive charge and most of its mass are concentrated. They deduced this by measuring how an alpha particle beam is scattered when it strikes a thin metal foil. The experiments were performed between 1908 and 1913 by Hans Geiger and Ernest Marsden under the direction of Ernest Rutherford at the Physical Laboratories of the University of Manchester.
Summary
Contemporary theories of atomic structure
The plum pudding model of the atom, as envisioned by Thomson.
The popular theory of atomic structure at the time of Rutherford's experiment was the "plum pudding model". This model was devised by Lord Kelvin and further developed by J. J. Thomson. Thomson was the scientist who discovered the electron,
and that it was a component of every atom. Thomson believed the atom
was a sphere of positive charge throughout which the electrons were
distributed, a bit like plums in a Christmas pudding. The existence of protons and neutrons was unknown at this time. They knew atoms were very tiny (Rutherford assumed they were in the order of 10−8 m in radius). This model was based entirely on classical (Newtonian) physics; the current accepted model uses quantum mechanics.
Thomson's model was not universally accepted even before
Rutherford's experiments. Thomson himself was never able to develop a
complete and stable model of his concept. Japanese scientist Hantaro Nagaoka rejected Thomson's model on the grounds that opposing charges cannot penetrate each other. He proposed instead that electrons orbit the positive charge like the rings around Saturn.
Implications of the plum pudding model
An alpha particle
is a sub-microscopic, positively charged particle of matter. According
to Thomson's model, if an alpha particle were to collide with an atom,
it would just fly straight through, its path being deflected by at most a
fraction of a degree. At the atomic scale, the concept of "solid
matter" is meaningless, so the alpha particle would not bounce off the
atom like a marble. It would be affected only by the atom's electric
fields, and Thomson's model predicted that the electric fields in an
atom are too weak to affect a passing alpha particle much (alpha
particles tend to move very fast). Both the negative and positive
charges within the Thomson atom are spread out over the atom's entire
volume. According to Coulomb's Law, the less concentrated a sphere of electric charge is, the weaker its electric field at its surface will be.
As
a worked example, consider an alpha particle passing tangentially to a
Thomson gold atom, where it will experience the electric field at its
strongest and thus experience the maximum deflection θ. Since the electrons are very light compared to the alpha particle, their influence can be neglected and the atom can be seen as a heavy sphere of positive charge.
- Qn = positive charge of gold atom = 79 e = 1.266×10−17 C
- Qα = charge of alpha particle = 2 e = 3.204×10−19 C
- r = radius of a gold atom = 1.44×10−10 m
- vα = velocity of alpha particle = 1.53×107 m/s
- mα = mass of alpha particle = 6.645×10−27 kg
- k = Coulomb's constant = 8.998×109 N·m2/C2
Using classical physics, the alpha particle's lateral change in momentum Δp can be approximated using the impulse of force relationship and the Coulomb force expression:
The above calculation is but an approximation of what happens when an
alpha particle comes near a Thomson atom, but it is clear that the
deflection at most will be in the order of a small fraction of a degree.
If the alpha particle were to pass through a gold foil some 400 atoms
thick and experience maximal deflection in the same direction
(unlikely), it would still be a small deflection.
The outcome of the experiments
Left: Had Thomson's model been correct, all the alpha particles should have passed through the foil with minimal scattering. Right: What Geiger and Marsden observed was that a small fraction of the alpha particles experienced strong deflection.
At Rutherford's behest, Geiger and Marsden performed a series of
experiments where they pointed a beam of alpha particles at a thin foil
of metal and measured the scattering pattern by using a fluorescent screen.
They spotted alpha particles bouncing off the metal foil in all
directions, some right back at the source. This should have been
impossible according to Thomson's model; the alpha particles should have
all gone straight through. Obviously, those particles had encountered
an electrostatic force far greater than Thomson's model suggested they
would, which in turn implied that the atom's positive charge was
concentrated in a much tinier volume than Thomson imagined.
When Geiger and Marsden shot alpha particles at their metal foil,
they noticed only a tiny fraction of the alpha particles were deflected
by more than 90°. Most flew straight through the foil. This suggested
that those tiny spheres of intense positive charge were separated by
vast gulfs of empty space.
Most particles passed through the empty space and experienced
negligible deviation, while a handful passed close to the nuclei of the
atoms and were deflected through large angles.
Rutherford thus rejected Thomson's model of the atom, and instead
proposed a model where the atom consisted of mostly empty space, with
all of its positive charge concentrated in its center in a very tiny
volume, surrounded by a cloud of electrons.
Timeline
Background
Ernest Rutherford was Langsworthy Professor of Physics at the Victoria University of Manchester (now the University of Manchester). He had already received numerous honours for his studies of radiation. He had discovered the existence of alpha rays, beta rays, and gamma rays, and had proved that these were the consequence of the disintegration of atoms. In 1906, he received a visit from a German physicist named Hans Geiger, and was so impressed that he asked Geiger to stay and help him with his research. Ernest Marsden was a physics undergraduate student studying under Geiger.
Alpha particles are tiny, positively charged particles that are spontaneously emitted by certain substances such as uranium and radium. Rutherford had discovered them in 1899. In 1908, he was trying to precisely measure their charge-to-mass ratio.
To do this, he first needed to know just how many alpha particles his
sample of radium was giving off (after which he would measure their
total charge and divide one by the other). Alpha particles are too tiny
to be seen with a microscope, but Rutherford knew that alpha particles
ionize air molecules, and if the air is within an electric field, the
ions will produce an electric current. On this principle, Rutherford and
Geiger designed a simple counting device which consisted of two
electrodes in a glass tube. Every alpha particle that passed through
the tube would create a pulse of electricity that could be counted. It
was an early version of the Geiger counter.
The counter that Geiger and Rutherford built proved unreliable
because the alpha particles were being too strongly deflected by their
collisions with the molecules of air within the detection chamber. The
highly variable trajectories of the alpha particles meant that they did
not all generate the same number of ions as they passed through the gas,
thus producing erratic readings. This puzzled Rutherford because he
had thought that alpha particles were just too heavy to be deflected so
strongly. Rutherford asked Geiger to investigate just how much matter
could scatter alpha rays.
The experiments they designed involved bombarding a metal foil
with alpha particles to observe how the foil scattered them in relation
to their thickness and material. They used a fluorescent screen to
measure the trajectories of the particles. Each impact of an alpha
particle on the screen produced a tiny flash of light. Geiger worked in a
darkened lab for hours on end, counting these tiny scintillations using
a microscope. Rutherford lacked the endurance for this work, which is why he left it to his younger colleagues. For the metal foil, they tested a variety of metals, but they preferred gold because they could make the foil very thin, as gold is very malleable. As a source of alpha particles, Rutherford's substance of choice was radon, a substance several million times more radioactive than uranium.
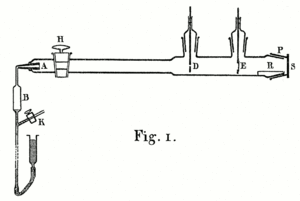
The 1908 experiment
This apparatus was described in a 1908 paper by Hans Geiger. It could only measure deflections of a few degrees.
A 1908 paper by Geiger, On the Scattering of α-Particles by Matter,
describes the following experiment. He constructed a long glass tube,
nearly two meters in length. At one end of the tube was a quantity of "radium emanation"
(R) that served as a source of alpha particles. The opposite end of
the tube was covered with a phosphorescent screen (Z). In the middle of
the tube was a 0.9 mm-wide slit. The alpha particles from R passed
through the slit and created a glowing patch of light on the screen. A
microscope (M) was used to count the scintillations on the screen and
measure their spread. Geiger pumped all the air out of the tube so that
the alpha particles would be unobstructed, and they left a neat and
tight image on the screen that corresponded to the shape of the slit.
Geiger then allowed some air in the tube, and the glowing patch became
more diffuse. Geiger then pumped out the air and placed some gold foil
over the slit at AA. This too caused the patch of light on the screen
to become more spread out. This experiment demonstrated that both air
and solid matter could markedly scatter alpha particles. The apparatus,
however, could only observe small angles of deflection. Rutherford
wanted to know if the alpha particles were being scattered by even
larger angles—perhaps larger than 90°.
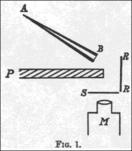
The 1909 experiment
In a 1909 paper, On a Diffuse Reflection of the α-Particles,
Geiger and Marsden described the experiment by which they proved that
alpha particles can indeed be scattered by more than 90°. In their
experiment, they prepared a small conical glass tube (AB) containing
"radium emanation" (radon), "radium A" (actual radium), and "radium C" (bismuth-214); its open end sealed with mica.
This was their alpha particle emitter. They then set up a lead plate
(P), behind which they placed a fluorescent screen (S). The tube was
held on the opposite side of plate, such that the alpha particles it
emitted could not directly strike the screen. They noticed a few
scintillations on the screen, because some alpha particles got around
the plate by bouncing off air molecules. They then placed a metal foil
(R) to the side of the lead plate. They pointed the tube at the foil to
see if the alpha particles would bounce off it and strike the screen on
the other side of the plate, and observed an increase in the number of
scintillations on the screen. Counting the scintillations, they observed
that metals with higher atomic mass, such as gold, reflected more alpha
particles than lighter ones such as aluminium.
Geiger and Marsden then wanted to estimate the total number of
alpha particles that were being reflected. The previous setup was
unsuitable for doing this because the tube contained several radioactive
substances (radium plus its decay products) and thus the alpha
particles emitted had varying ranges,
and because it was difficult for them to ascertain at what rate the
tube was emitting alpha particles. This time, they placed a small
quantity of radium C (bismuth-214) on the lead plate, which bounced off a
platinum reflector (R) and onto the screen. They found that only a
tiny fraction of the alpha particles that struck the reflector bounced
onto the screen (in this case, 1 in 8000).
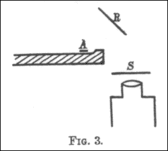
The 1910 experiment
This
apparatus was described in 1910 paper by Geiger. It was designed to
precisely measure how the scattering varied according to the substance
and thickness of the foil.
A 1910 paper by Geiger, The Scattering of the α-Particles by Matter,
describes an experiment by which he sought to measure how the most
probable angle through which an a-particle is deflected varies with the
material it passes through, the thickness of said material, and the
velocity of the alpha particles. He constructed an airtight glass tube
from which the air was pumped out. At one end was a bulb (B) containing
"radium emanation" (radon-222). By means of mercury, the radon in B was pumped up the narrow glass pipe whose end at A was plugged with mica. At the other end of the tube was a fluorescent zinc sulfide
screen (S). The microscope which he used to count the scintillations
on the screen was affixed to a vertical millimeter scale with a vernier,
which allowed Geiger to precisely measure where the flashes of light
appeared on the screen and thus calculate the particles' angles of
deflection. The alpha particles emitted from A was narrowed to a beam by
a small circular hole at D. Geiger placed a metal foil in the path of
the rays at D and E to observe how the zone of flashes changed. He
could also vary the velocity of the alpha particles by placing extra
sheets of mica or aluminium at A.
From the measurements he took, Geiger came to the following conclusions:
- the most probable angle of deflection increases with the thickness of the material
- the most probable angle of deflection is proportional to the atomic mass of the substance
- the most probable angle of deflection decreases with the velocity of the alpha particles
- the probability that a particle will be deflected by more than 90° is vanishingly small
Rutherford mathematically models the scattering pattern
Considering
the results of the above experiments, Rutherford published a landmark
paper in 1911 titled "The Scattering of α and β Particles by Matter and
the Structure of the Atom" wherein he proposed that the atom contains at
its center a volume of electric charge that is very small and intense
(in fact, Rutherford treats it as a point charge in his calculations).
For the purpose of his mathematical calculations he assumed this
central charge was positive, but he admitted he could not prove this and
that he had to wait for other experiments to develop his theory.
Rutherford developed a mathematical equation that modeled how the
foil should scatter the alpha particles if all the positive charge and
most of the atomic mass was concentrated in a single point at the center
of an atom.
- s = the number of alpha particles falling on unit area at an angle of deflection Φ
- r = distance from point of incidence of α rays on scattering material
- X = total number of particles falling on the scattering material
- n = number of atoms in a unit volume of the material
- t = thickness of the foil
- Qn = positive charge of the atomic nucleus
- Qα = positive charge of the alpha particles
- m = mass of an alpha particle
- v = velocity of the alpha particle
From the scattering data, Rutherford estimated the central charge Qn to be about +100 units.
The 1913 experiment
In a 1913 paper, The Laws of Deflexion of α Particles through Large Angles,
Geiger and Marsden describe a series of experiments by which they
sought to experimentally verify the above equation that Rutherford
developed. Rutherford's equation predicted that the number of
scintillations per minute s that will be observed at a given angle Φ should be proportional to:
- csc4(Φ/2)
- thickness of foil t
- magnitude of central charge Qn
- 1/(mv2)2
Their 1913 paper describes four experiments by which they proved each of these four relationships.
This
apparatus was described in a 1913 paper by Geiger and Marsden. It was
designed to accurately measure the scattering pattern of the alpha
particles produced by the metal foil (F). The microscope (M) and screen
(S) were affixed to a rotating cylinder and could be moved a full circle
around the foil so that they could count scintillations from every
angle.
To test how the scattering varied with the angle of deflection (i.e. if s ∝ csc4(Φ/2))
Geiger and Marsden built an apparatus that consisted of a hollow metal
cylinder mounted on a turntable. Inside the cylinder was a metal foil
(F) and a radiation source containing radon (R), mounted on a detached
column (T) which allowed the cylinder to rotate independently. The
column was also a tube by which air was pumped out of the cylinder. A
microscope (M) with its objective lens covered by a fluorescent zinc sulfide
screen (S) penetrated the wall of the cylinder and pointed at the metal
foil. By turning the table, the microscope could be moved a full circle
around the foil, allowing Geiger to observe and count alpha particles
deflected by up to 150°. Correcting for experimental error, Geiger and
Marsden found that the number of alpha particles that are deflected by a
given angle Φ is indeed proportional to csc4(Φ/2).
Geiger and Marsden then tested how the scattering varied with the thickness of the foil (i.e. if s ∝ t).
They constructed a disc (S) with six holes drilled in it. The holes
were covered with metal foil (F) of varying thickness, or none for
control. This disc was then sealed in a brass ring (A) between two
glass plates (B and C). The disc could be rotated by means of a rod (P)
to bring each window in front of the alpha particle source (R). On the
rear glass pane was a zinc sulfide
screen (Z). Geiger and Marsden found that the number of scintillations
that appeared on the zinc sulfide screen was indeed proportional to the
thickness as long as said thickness was small.
Geiger and Marsden reused the above apparatus to measure how the
scattering pattern varied with the square of the nuclear charge (i.e. if
s ∝ Qn2). Geiger and Marsden didn't know
what the positive charge of the nucleus of their metals were (they had
only just discovered the nucleus existed at all), but they assumed it
was proportional to the atomic weight, so they tested whether the
scattering was proportional to the atomic weight squared. Geiger and
Marsden covered the holes of the disc with foils of gold, tin, silver,
copper, and aluminum. They measured each foil's stopping power by
equating it to an equivalent thickness of air. They counted the number
of scintillations per minute that each foil produced on the screen. They
divided the number of scintillations per minute by the respective
foil's air equivalent, then divided again by the square root of the
atomic weight (Geiger and Marsden knew that for foils of equal stopping
power, the number of atoms per unit area is proportional to the square
root of the atomic weight). Thus, for each metal, Geiger and Marsden
obtained the number of scintillations that a fixed number of atoms
produce. For each metal, they then divided this number by the square of
the atomic weight, and found that the ratios were more or less the same.
Thus they proved that s ∝ Qn2.
Finally, Geiger and Marsden tested how the scattering varied with the velocity of the alpha particles (i.e. if s ∝ 1/v4). Using the same apparatus again, they slowed the alpha particles by placing extra sheets of mica
in front of the alpha particle source. They found that, within the
range of experimental error, that the number of scinitillations was
indeed proportional to 1/v4.
Rutherford determines the nucleus is positively charged
In his 1911 paper,
Rutherford assumed that the central charge of the atom was positive,
but a negative charge would have fitted his scattering model just as
well. In a 1913 paper,
Rutherford declared that the "nucleus" (as he now called it) was indeed
positively charged, based on the result of experiments exploring the
scattering of alpha particles in various gases.
In 1917, Rutherford and his assistant William Kay began exploring
the passage of alpha particles through gases such as hydrogen and
nitrogen. In an experiment where they shot a beam of alpha particles
through hydrogen, the alpha particles knocked the hydrogen nuclei
forwards in the direction of the beam, not backwards. In an experiment
where they shot alpha particles through nitrogen, he discovered that the
alpha particles knocked hydrogen nuclei (i.e. protons) out of the
nitrogen nuclei.
Legacy
When Geiger reported to Rutherford that he had spotted alpha
particles being strongly deflected, Rutherford was astounded. In a
lecture Rutherford delivered at Cambridge University, he said:
It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you. On consideration, I realized that this scattering backward must be the result of a single collision, and when I made calculations I saw that it was impossible to get anything of that order of magnitude unless you took a system in which the greater part of the mass of the atom was concentrated in a minute nucleus. It was then that I had the idea of an atom with a minute massive centre, carrying a charge.
— Ernest Rutherford
Accolades soon flooded in. Hantaro Nagaoka,
who had once proposed a Saturnian model of the atom, wrote to
Rutherford from Tokyo in 1911: "Congratulations on the simpleness of the
apparatus you employ and the brilliant results you obtained". The
conclusions of these experiments revealed how all matter on Earth is
structured and thus affected every scientific and engineering
discipline, making it one of the most pivotal scientific discoveries of
all time. The astronomer Arthur Eddington called Rutherford's discovery the most important scientific achievement since Democritus proposed the atom ages earlier.
Like most scientific models, Rutherford's atomic model was neither perfect nor complete. According to classical Newtonian physics,
it was in fact impossible. Accelerating charged particles radiate
electromagnetic waves, so an electron orbiting an atomic nucleus in
theory would spiral into the nucleus as it loses energy. To fix this
problem, scientists had to incorporate quantum mechanics into
Rutherford's model.