The sulfur cycle is the collection of processes by which sulfur moves between rocks, waterways and living systems. Such biogeochemical cycles are important in geology because they affect many minerals. Biochemical cycles are also important for life because sulfur is an essential element, being a constituent of many proteins and cofactors, and sulfur compounds can be used as oxidants or reductants in microbial respiration. The global sulfur
cycle involves the transformations of sulfur species through different
oxidation states, which play an important role in both geological and
biological processes.
The Sulfur cycle (in general)
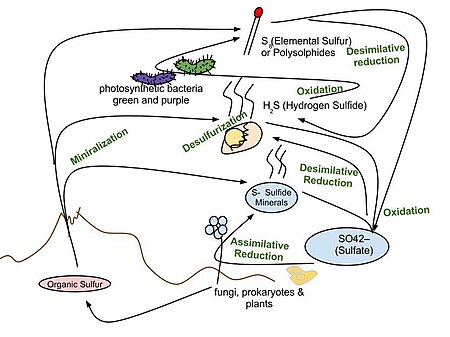
Sulfur cycle
Steps of the sulfur cycle are:
- Mineralization of organic sulfur into inorganic forms, such as hydrogen sulfide (H2S), elemental sulfur, as well as sulfide minerals.
- Oxidation of hydrogen sulfide, sulfide, and elemental sulfur (S) to sulfate (SO42−).
- Reduction of sulfate to sulfide.
- Incorporation of sulfide into organic compounds (including metal-containing derivatives).
Structure of 3'-phosphoadenosine-5'-phosphosulfate, a key intermediate in the sulfur cycle.
These are often termed as follows:
- Assimilative sulfate reduction in which sulfate (SO42−) is reduced by plants, fungi and various prokaryotes. The oxidation states of sulfur are +6 in sulfate and –2 in R–SH.
- Desulfurization in which organic molecules containing sulfur can be desulfurized, producing hydrogen sulfide gas (H2S, oxidation state = –2). An analogous process for organic nitrogen compounds is deamination.
- Oxidation of hydrogen sulfide produces elemental sulfur (S8), oxidation state = 0. This reaction occurs in the photosynthetic green and purple sulfur bacteria and some chemolithotrophs. Often the elemental sulfur is stored as polysulfides.
- Oxidation in elemental sulfur by sulfur oxidizers produces sulfate.
- Dissimilative sulfur reduction in which elemental sulfur can be reduced to hydrogen sulfide.
- Dissimilative sulfate reduction in which sulfate reducers generate hydrogen sulfide from sulfate.
Sulfur oxidation state
Sulfur
has four main oxidation states in nature, which are -2, +2, +4, and +6.
The common sulfur species of each oxidation state are listed as
follows:
- S2-: H2S, FeS, FeS2, CuS
- S0: native, or elemental, sulfur
- S2+: SO
- S4+: SO2, sulfite (SO32-)
- S6+: SO42- (H2SO4, CaSO4), SF6
Sulfur sources and sinks
Sulfur is found in oxidation states ranging from +6 in SO42− to -2 in sulfides.
Thus, elemental sulfur can either give or receive electrons depending
on its environment. On the anoxic early Earth, most sulfur was present
in minerals such as pyrite (FeS2). Over Earth history, the amount of mobile sulfur increased through volcanic activity as well as weathering of the crust in an oxygenated atmosphere. Earth's main sulfur sink is the oceans SO42−, where it is the major oxidizing agent.
When SO42− is assimilated by organisms, it is reduced and converted to organic sulfur, which is an essential component of proteins. However, the biosphere does not act as a major sink for sulfur, instead the majority of sulfur is found in seawater or sedimentary rocks including: pyrite rich shales, evaporite rocks (anhydrite and baryte), and calcium and magnesium carbonates (i.e. carbonate-associated sulfate). The amount of sulfate in the oceans is controlled by three major processes:
- 1. input from rivers
- 2. sulfate reduction and sulfide re-oxidation on continental shelves and slopes
- 3. burial of anhydrite and pyrite in the oceanic crust.
The primary natural source of sulfur to the atmosphere is sea spray or windblown sulfur rich dust, neither of which is long lived in the atmosphere. In recent times, the large annual input of sulfur from the burning of coal and other fossil fuels has added a substantial amount SO2 which acts as an air pollutant. In the geologic past, igneous intrusions into coal measures
have caused large scale burning of these measures, and consequential
release of sulfur to the atmosphere. This has led to substantial
disruption to the climate system, and is one of the proposed causes of
the Permian–Triassic extinction event.
Dimethylsulfide [(CH3)2S or DMS] is produced by the decomposition of dimethylsulfoniopropionate (DMSP) from dying phytoplankton cells in the ocean's photic zone,
and is the major biogenic gas emitted from the sea, where it is
responsible for the distinctive “smell of the sea” along coastlines.
DMS is the largest natural source of sulfur gas, but still only has a
residence time of about one day in the atmosphere and a majority of it
is redeposited in the oceans rather than making it to land. However, it
is a significant factor in the climate system, as it is involved in the
formation of clouds.
Biologically and thermochemically driven sulfate reduction
Sulfur can be reduced both biologically and thermochemically. Dissimilatory sulfate reduction has two different definitions:
- 1. the microbial process that converts sulfate to sulfide for energy gain, and
- 2. a set of forward and reverse pathways that progress from the uptake and release of sulfate by the cell to its conversion to various sulfur intermediates, and ultimately to sulfide which is released from the cell.
Sulfide and thiosulfate are the most abundant reduced inorganic
sulfur species in the environments and are converted to sulfate,
primarily by bacterial action, in the oxidative half of the sulfur
cycle.
Bacterial sulfate reduction (BSR) can only occur at temperature from 0
up to 60–80 °C because above that temperature almost all
sulfate-reducing microbes can no longer metabolize. Few microbes can
form H2S at higher temperatures but appear to be very rare
and do not metabolize in settings where normal bacterial sulfate
reduction is occurring. Bacterial sulfate reduction is geologically
instantaneous happening on the order of hundreds to thousands of years.
Thermochemical sulfate reduction (TSR) occurs at much higher
temperatures (160–180 °C) and over longer time intervals, several tens
of thousands to a few million years.
The main difference between these two reactions is obvious, one
is organically driven and the other is chemically driven. Therefore,
the temperature for thermochemical sulfate reduction is much higher due
to the activation energy required to reduce sulfate.
Bacterial sulfate reductions requires lower temperatures because the
sulfur reducing bacteria can only live at relatively low temperature
(below 60 °C). Bacterial sulfate reduction also requires a relatively
open system; otherwise the bacteria will poison themselves when the
sulfate levels rise above 5–10%.
The organic reactants involved in bacterial sulfate reduction are
organic acids which are distinctive from the organic reactants needed
for thermochemical sulfate reduction. In both cases sulfate is usually
derived from the dissolution of gypsum or taken directly out of the seawater.
The factors that control whether bacterial sulfate reduction or
thermochemical sulfate reduction will occur are temperature, which is
generally a product of depth, with bacterial sulfate reduction occurring
in shallower levels than thermochemical sulfate reduction. Their solid
products are similar but can be distinguished from one another petrographically, due to their differing crystal sizes, shapes and reflectivity.
Sulfur-oxidizing bacteria in hydrothermal vents
Hydrothermal vents emit hydrogen sulfide that support the carbon fixation of chemolithotrophic bacteria that oxidize hydrogen sulfide with oxygen to produce elemental sulfur or sulfate. The chemical reactions are as follows:
CO2 + 4H2S + O2 -> CH2O + 4S0 + 3H2O
CO2 + H2S + O2 + H2O -> CH2O + SO42- + 2H+
In modern oceans, Thiomicrospira, Halothiobacillus, and Beggiatoa are primary sulfur oxidizing bacteria, and form chemosynthetic symbioses with animal hosts. The host provides metabolic substrates (e.g., CO2, O2, H2O)
to the symbiont while the symbiont generates organic carbon for
sustaining the metabolic activities of the host. The produced sulfate
usually combines with the leached calcium ions to form gypsum, which can form widespread deposits on near mid-ocean spreading centers.
δ34S
Although 25 isotopes are known for sulfur, only four are stable and of geochemical importance. Of those four, two (32S, light and 34S, heavy) comprise (99.22%) of S on Earth. The vast majority (95.02%) of S occurs as 32S with only 4.21% in 34S. The ratio of these two isotopes is fixed in our solar system
and has been since its formation. The bulk Earth sulfur isotopic ratio
is thought to be the same as the ratio of 22.22 measured from the Canyon Diablo troilite (CDT), a meteorite. That ratio is accepted as the international standard and is therefore set at δ0.00. Deviation from 0.00 is expressed as the δ34S which is a ratio in per mill (‰). Positive values correlate to increased levels of 34S, whereas negative values correlate with greater 32S in a sample.
Formation of sulfur minerals through non-biogenic processes does
not substantially differentiate between the light and heavy isotopes,
therefore sulfur isotope ratios in gypsum or barite
should be the same as the overall isotope ratio in the water column at
their time of precipitation. Sulfate reduction through biologic
activity strongly differentiates between the two isotopes because of the
more rapid enzymic reaction with 32S.
Sulfate metabolism results in an isotopic depletion of -18‰, and
repeated cycles of oxidation and reduction can result in values up to
-50 ‰. Average present day seawater values of δ34S are on the order of +21‰.
Throughout geologic history the sulfur cycle and the isotopic
ratios have coevolved with the biosphere becoming overall more negative
with the increases in biologically driven sulfate reduction, but also
show substantial positive excursion. In general positive excursions in
the sulfur isotopes mean that there is an excess of pyrite deposition
rather than oxidation of sulfide minerals exposed on land.
Marine sulfur cycle
The sulfur cycle in marine environments has been well-studied via the tool of sulfur isotope systematics expressed as δ34S. The modern global oceans have sulfur storage of 1.3 × 1021 g, mainly occurring as sulfate with the δ34S value of +21‰. The overall input flux is 1.0 × 1014 g/year with the sulfur isotope composition of ~3‰. Riverine sulfate derived from the terrestrial weathering of sulfide minerals (δ34S
= +6‰) is the primary input of sulfur to the oceans. Other sources are
metamorphic and volcanic degassing and hydrothermal activity (δ34S = 0‰), which release reduced sulfur species (e.g., H2S and S0).
There are two major outputs of sulfur from the oceans. The first sink
is the burial of sulfate either as marine evaporites (e.g., gypsum) or
carbonate-associated sulfate (CAS), which accounts for 6 × 1013 g/year (δ34S = +21‰). The second sulfur sink is pyrite burial in shelf sediments or deep seafloor sediments (4 × 1013 g/year; δ34S = -20‰). The total marine sulfur output flux is 1.0 × 1014 g/year which matches the input fluxes, implying the modern marine sulfur budget is at steady state. The residence time of sulfur in modern global oceans is 13,000,000 year.
Evolution of the sulfur cycle
The isotopic composition of sedimentary sulfides provides primary information on the evolution of the sulfur cycle.
The total inventory of sulfur compounds on the surface of the Earth (nearly 1022 g S) represents the total outgassing of sulfur through geologic time.
Rocks analyzed for sulfur content are generally organic-rich shales
meaning they are likely controlled by biogenic sulfur reduction.
Average seawater curves are generated from evaporites deposited
throughout geologic time because again, since they do not discriminate
between the heavy and light sulfur isotopes, they should mimic the ocean
composition at the time of deposition.
4.6 billion years ago (Ga) the Earth formed and had a theoretical δ34S value of 0. Since there was no biologic activity on early Earth there would be no isotopic fractionation.
All sulfur in the atmosphere would be released during volcanic
eruptions. When the oceans condensed on Earth, the atmosphere was
essentially swept clean of sulfur gases, owing to their high solubility
in water. Throughout the majority of the Archean
(4.6–2.5 Ga) most systems appeared to be sulfate-limited. Some small
Archean evaporite deposits require that at least locally elevated
concentrations (possibly due to local volcanic activity) of sulfate
existed in order for them to be supersaturated and precipitate out of
solution.
3.8–3.6 Ga marks the beginning of the exposed geologic record because this is the age of the oldest rocks on Earth. Metasedimentary
rocks from this time still have an isotopic value of 0 because the
biosphere was not developed enough (possibly at all) to fractionate
sulfur.
3.5 Ga anoxyogenic photosynthesis is established and provides a weak source of sulfate to the global ocean with sulfate concentrations incredibly low the δ34S is still basically 0. Shortly after, at 3.4 Ga the first evidence for minimal fractionation in evaporitic sulfate in association with magmatically derived sulfides can be seen in the rock record. This fractionation shows possible evidence for anoxygenic phototrophic bacteria.
2.8 Ga marks the first evidence for oxygen production through
photosynthesis. This is important because there cannot be sulfur
oxidation without oxygen in the atmosphere. This exemplifies the
coevolution of the oxygen and sulfur cycles as well as the biosphere.
2.7–2.5 Ga is the age of the oldest sedimentary rocks to have a depleted δ 34S which provide the first compelling evidence for sulfate reduction.
2.3 Ga sulfate increases to more than 1 mM; this increase in sulfate is coincident with the "Great Oxygenation Event",
when redox conditions on Earth's surface are thought by most workers to
have shifted fundamentally from reducing to oxidizing.
This shift would have led to an incredible increase in sulfate
weathering which would have led to an increase in sulfate in the oceans.
The large isotopic fractionations that would likely be associated with
bacteria reduction are produced for the first time. Although there was a
distinct rise in seawater sulfate at this time it was likely still only
less than 5–15% of present-day levels.
At 1.8 Ga, Banded iron formations (BIF) are common sedimentary rocks throughout the Archean and Paleoproterozoic; their disappearance marks a distinct shift in the chemistry of ocean water. BIFs have alternating layers of iron oxides and chert. BIFs only form if the water is allowed to supersaturate in dissolved iron (Fe2+) meaning there cannot be free oxygen or sulfur in the water column because it would form Fe3+
(rust) or pyrite and precipitate out of solution. Following this
supersaturation, the water must become oxygenated in order for the
ferric rich bands to precipitate it must still be sulfur poor otherwise
pyrite would form instead of Fe3+. It has been hypothesized
that BIFs formed during the initial evolution of photosynthetic
organisms that had phases of population growth, causing over production
of oxygen. Due to this over production they would poison themselves
causing a mass die off, which would cut off the source of oxygen and
produce a large amount of CO2 through the decomposition of
their bodies, allowing for another bacterial bloom. After 1.8 Ga sulfate
concentrations were sufficient to increase rates of sulfate reduction
to greater than the delivery flux of iron to the oceans.
Along with the disappearance of BIF, the end of the Paleoproterozoic
also marks the first large scale sedimentary exhalative deposits
showing a link between mineralization and a likely increase in the
amount of sulfate in sea water. In the Paleoproterozoic the sulfate in
seawater had increased to an amount greater than in the Archean, but was
still lower than present day values. The sulfate levels in the Proterozoic
also act as proxies for atmospheric oxygen because sulfate is produced
mostly through weathering of the continents in the presence of oxygen.
The low levels in the Proterozoic simply imply that levels of
atmospheric oxygen fell between the abundances of the Phanerozoic and
the deficiencies of the Archean.
750 million years ago (Ma) there is a renewed deposition of BIF
which marks a significant change in ocean chemistry. This was likely
due to snowball earth episodes where the entire globe including the oceans was covered in a layer of ice cutting off oxygenation. In the late Neoproterozoic
high carbon burial rates increased the atmospheric oxygen level to more than 10% of its present-day value. In the Latest Neoproterozoic another
major oxidizing event occurred on Earth's surface that resulted in an
oxic deep ocean and possibly allowed for the appearance of multicellular
life.
During the last 600 million years, seawater SO4 has varied between +10 and +30‰ in δ34S,
with an average value close to that of today. This coincides with
atmospheric O2 levels reaching something close to modern values around
the Precambrian–Cambrian boundary.
Over a shorter time scale (ten million years) changes in the
sulfur cycle are easier to observe and can be even better constrained
with oxygen isotopes. Oxygen is continually incorporated into the
sulfur cycle through sulfate oxidation and then released when that
sulfate is reduced once again.
Since different sulfate sources within the ocean have distinct oxygen
isotopic values it may be possible to use oxygen to trace the sulfur
cycle. Biological sulfate reduction preferentially selects lighter
oxygen isotopes for the same reason that lighter sulfur isotopes are
preferred. By studying oxygen isotopes in ocean sediments over the last
10 million years were able to better constrain the sulfur concentrations in sea water through that same time. They found that the sea level changes due to Pliocene and Pleistocene glacial
cycles changed the area of continental shelves which then disrupted the
sulfur processing, lowering the concentration of sulfate in the sea
water. This was a drastic change as compared to preglacial times before
2 million years ago.
The Great Oxidation Event and sulfur isotope mass-independent fractionation
The Great Oxygenation Event (GOE) is characterized by the disappearance of sulfur isotope mass-independent fractionation (MIF) in the sedimentary records at around 2.45 billion years ago (Ga). The MIF of sulfur isotope (Δ33S) is defined by the deviation of measured δ33S value from the δ33S value inferred from the measured δ34S
value according to the mass dependent fractionation law. The Great
Oxidation Event represented a massive transition of global sulfur
cycles. Before the Great Oxidation Event, the sulfur cycle was heavily
influenced by the ultraviolet (UV) radiation and the associated photochemical reactions, which induced the sulfur isotope mass-independent fractionation (Δ33S ≠ 0). The preservation of sulfur isotope mass-independent fractionation signals requires the atmospheric O2 lower than 10−5 of present atmospheric level (PAL). The disappearance of sulfur isotope mass-independent fractionation at ~2.45 Ga indicates that atmospheric pO2 exceeded 10−5 present atmospheric level after the Great Oxygenation Event.
Oxygen played an essential role in the global sulfur cycles after the
Great Oxygenation Event, such as oxidative weathering of sulfides. The burial of pyrite in sediments in turn contributes to the accumulation of free O2 in Earth's surface environment.
Economic importance
Sulfur is intimately involved in production of fossil fuels
and a majority of metal deposits because of its ability to act as an
oxidizing or reducing agent. The vast majority of the major mineral
deposits on Earth contain a substantial amount of sulfur including, but
not limited to: sedimentary exhalative deposits (SEDEX), Carbonate-hosted lead-zinc ore deposits (Mississippi Valley-Type MVT) and porphyry copper deposits. Iron sulfides, galena and sphalerite will form as by-products of hydrogen sulfide generation, as long as the respective transition or base metals are present or transported to a sulfate reduction site.
If the system runs out of reactive hydrocarbons economically viable
elemental sulfur deposits may form. Sulfur also acts as a reducing
agent in many natural gas reservoirs and generally ore forming fluids
have a close relationship with ancient hydrocarbon seeps or vents.
Important sources of sulfur in ore deposits are generally
deep-seated, but they can also come from local country rocks, sea water,
or marine evaporites.
The presence or absence of sulfur is one of the limiting factors on
both the concentration of precious metals and its precipitation from
solution. pH, temperature and especially redox
states determine whether sulfides will precipitate. Most sulfide
brines will remain in concentration until they reach reducing
conditions, a higher pH or lower temperatures.
Ore fluids are generally linked to metal rich waters that have been heated within a sedimentary basin under the elevated thermal conditions typically in extensional tectonic settings. The redox conditions of the basin lithologies
exert an important control on the redox state of the metal-transporting
fluids and deposits can form from both oxidizing and reducing fluids.
Metal-rich ore fluids tend to be by necessity comparatively sulfide
deficient, so a substantial portion of the sulfide must be supplied from
another source at the site of mineralization. Bacterial reduction of
seawater sulfate or a euxinic (anoxic and H2S-containing) water column is a necessary source of that sulfide. When present, the δ34S values of barite
are generally consistent with a seawater sulfate source, suggesting
baryte formation by reaction between hydrothermal barium and sulfate in
ambient seawater.
Once fossil fuels or precious metals are discovered and either
burned or milled, the sulfur become a waste product which must be dealt
with properly or it can become a pollutant. There has been a great
increase in the amount of sulfur in our present day atmosphere because
of the burning of fossil fuels. Sulfur acts as a pollutant and an
economic resource at the same time.
Human impact
Human activities have a major effect on the global sulfur cycle. The burning of coal, natural gas, and other fossil fuels
has greatly increased the amount of S in the atmosphere and ocean and
depleted the sedimentary rock sink. Without human impact sulfur would
stay tied up in rocks for millions of years until it was uplifted
through tectonic events and then released through erosion and weathering
processes. Instead it is being drilled, pumped and burned at a
steadily increasing rate. Over the most polluted areas there has been a
30-fold increase in sulfate deposition.
Although the sulfur curve shows shifts between net sulfur
oxidation and net sulfur reduction in the geologic past, the magnitude
of the current human impact is probably unprecedented in the geologic
record. Human activities greatly increase the flux of sulfur to the atmosphere, some of which is transported globally. Humans are mining coal and extracting petroleum from the Earth's crust at a rate that mobilizes 150 x 1012 gS/yr, which is more than double the rate of 100 years ago. The result of human impact on these processes is to increase the pool of oxidized sulfur (SO4)
in the global cycle, at the expense of the storage of reduced sulfur in
the Earth's crust. Therefore, human activities do not cause a major
change in the global pools of S, but they do produce massive changes in
the annual flux of S through the atmosphere.
When SO2 is emitted as an air pollutant, it forms sulfuric acid through reactions with water in the atmosphere. Once the acid is completely dissociated in water the pH can drop to 4.3 or lower causing damage to both man-made and natural systems. According to the EPA, acid rain
is a broad term referring to a mixture of wet and dry deposition
(deposited material) from the atmosphere containing higher than normal
amounts of nitric and sulfuric acids. Distilled water (water without
any dissolved constituents), which contains no carbon dioxide,
has a neutral pH of 7. Rain naturally has a slightly acidic pH of 5.6,
because carbon dioxide and water in the air react together to form
carbonic acid, a very weak acid. Around Washington, D.C., however, the
average rain pH is between 4.2 and 4.4. Since pH is on a log scale
dropping by 1 (the difference between normal rain water and acid rain)
has a dramatic effect on the strength of the acid. In the United
States, roughly 2/3 of all SO2 and 1/4 of all NO3 come from electric power generation that relies on burning fossil fuels, like coal.