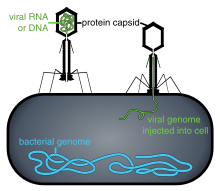
Phage injecting its genome into bacterial cell
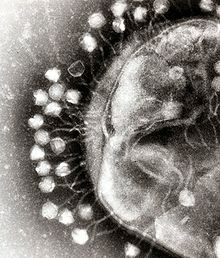
An electron micrograph of bacteriophages attached to a bacterial cell. These viruses are the size and shape of coliphage T1.
Phage therapy or viral phage therapy is the therapeutic use of bacteriophages to treat pathogenic bacterial infections. Bacteriophages, known as phages, are a form of viruses. Phages attach to bacterial cells, and inject a viral genome into the cell. The viral genome effectively replaces the bacterial genome, halting the bacterial infection. The bacterial cell causing the infection is unable to reproduce, and instead produces additional phages. Phages are very selective in the strains of bacteria they are effective against. Advantages include reduced side-effects and reduced risk of the bacterial developing resistance. Disadvantages include the difficulty of finding an effective phage for a particular infection.
Phages are often compared to antibiotics. Phages tend to be more successful than antibiotics where there is a biofilm covered by a polysaccharide layer, which antibiotics typically cannot penetrate. Bacteriophages are much more specific than antibiotics. They are typically harmless not only to the host organism but also to other beneficial bacteria, such as the gut flora, reducing the chances of opportunistic infections. They have a high therapeutic index, that is, phage therapy would be expected to give rise to few side effects, even at higher-than therapeutic levels. Because phages replicate in vivo (in cells of living organism), a smaller effective dose can be used.
This specificity is also a disadvantage: a phage will kill a bacterium only if it matches the specific strain. Consequently, phage mixtures ("cocktails") are often used to improve the chances of success.
Alternatively, samples taken from recovering patients sometimes
contain appropriate phages that can be grown to cure other patients
infected with the same strain.
Phages are currently being used therapeutically to treat
bacterial infections that do not respond to conventional antibiotics,
particularly in Russia and Georgia. There is also a phage therapy unit in Wrocław, Poland, established 2005, the only such centre in a European Union country. Phages are the subject of renewed clinical attention in western countries, such as the United States. In 2019, the United States Food and Drug Administration approved the first US clinical trial for intravenous phage therapy.
Phage therapy has many potential applications in human medicine as well as dentistry, veterinary science, and agriculture. If the target host of a phage therapy treatment is not an animal, the term "biocontrol" (as in phage-mediated biocontrol of bacteria) is usually employed, rather than "phage therapy".
History
Félix d'Hérelle, discoverer of phage therapy
Phage in action on cultured Bacillus anthracis.
The discovery of bacteriophages was reported by the Englishman Frederick Twort in 1915 and the French-Canadian Felix d'Hérelle in 1917. D'Hérelle said that the phages always appeared in the stools of Shigella dysentery patients shortly before they began to recover.
He "quickly learned that bacteriophages are found wherever bacteria
thrive: in sewers, in rivers that catch waste runoff from pipes, and in
the stools of convalescent patients".
Phage therapy was immediately recognized by many to be a key way
forward for the eradication of pathogenic bacterial infections. A
Georgian, George Eliava, was making similar discoveries. He travelled to the Pasteur Institute in Paris where he met d'Hérelle, and in 1923 he founded the Eliava Institute in Tbilisi, Georgia, devoted to the development of phage therapy. Phage therapy is used in Russia, Georgia and Poland.
In Russia, extensive research and development soon began in this
field. In the United States during the 1940s commercialization of phage
therapy was undertaken by Eli Lilly and Company.
While knowledge was being accumulated regarding the biology of
phages and how to use phage cocktails correctly, early uses of phage
therapy were often unreliable. Since the early 20th century, research into the development of viable therapeutic antibiotics had also been underway, and by 1942 the antibiotic penicillin G
had been successfully purified and saw use during the Second World War.
The drug proved to be extraordinarily effective in the treatment of
injured Allied soldiers whose wounds had become infected. By 1944,
large-scale production of Penicillin had been made possible, and in 1945
it became publicly available in pharmacies. Due to the drug's success,
it was marketed widely in the U.S. and Europe, leading Western
scientists to mostly lose interest in further use and study of phage
therapy for some time.
Isolated from Western advances in antibiotic production in the
1940s, Russian scientists continued to develop already successful phage
therapy to treat the wounds of soldiers in field hospitals. During World War II,
the Soviet Union used bacteriophages to treat many soldiers infected
with various bacterial diseases e.g. dysentery and gangrene. Russian
researchers continued to develop and to refine their treatments and to
publish their research and results. However, due to the scientific
barriers of the Cold War, this knowledge was not translated and did not proliferate across the world.
A summary of these publications was published in English in 2009 in "A
Literature Review of the Practical Application of Bacteriophage
Research".
There is an extensive library and research center at the George Eliava Institute in Tbilisi, Georgia. Phage therapy is today a widespread form of treatment in that region.
As a result of the development of antibiotic resistance
since the 1950s and an advancement of scientific knowledge, there has
been renewed interest worldwide in the ability of phage therapy to
eradicate bacterial infections and chronic polymicrobial biofilm (including in industrial situations).
Phages have been investigated as a potential means to eliminate pathogens like Campylobacter in raw food and Listeria in fresh food or to reduce food spoilage bacteria. In agricultural practice phages were used to fight pathogens like Campylobacter, Escherichia and Salmonella in farm animals, Lactococcus and Vibrio pathogens in fish from aquaculture and Erwinia and Xanthomonas
in plants of agricultural importance. The oldest use was, however, in
human medicine. Phages have been used against diarrheal diseases caused
by E. coli, Shigella or Vibrio and against wound infections caused by facultative pathogens of the skin like staphylococci and streptococci.
Recently the phage therapy approach has been applied to systemic and
even intracellular infections and the addition of non-replicating phage
and isolated phage enzymes like lysins to the antimicrobial arsenal. However, actual proof for the efficacy of these phage approaches in the field or the hospital is not available.
Some of the interest in the West can be traced back to 1994, when
Soothill demonstrated (in an animal model) that the use of phages could
improve the success of skin grafts by reducing the underlying Pseudomonas aeruginosa infection. Recent studies have provided additional support for these findings in the model system.
Although not "phage therapy" in the original sense, the use of
phages as delivery mechanisms for traditional antibiotics constitutes
another possible therapeutic use. The use of phages to deliver antitumor agents has also been described in preliminary in vitro experiments for cells in tissue culture.
In June 2015 the European Medicines Agency hosted a one-day workshop on the therapeutic use of bacteriophages
and in July 2015 the National Institutes of Health (USA) hosted a
two-day workshop "Bacteriophage Therapy: An Alternative Strategy to
Combat Drug Resistance".
In 2017, a pair of genetically engineered
phages along with one naturally occurring (so-called "phage Muddy")
each from among those catalogued by Science Education Alliance-Phages
Hunters Advancing Genomics and Evolutionary Science (SEA-PHAGES) at the Howard Hughes Medical Institute by Graham Hatfull and colleagues, was used by microbiologist James Soothill at Great Ormond Street Hospital for Children in London to treat an antibiotic-resistant bacterial (Mycobacterium abscessus) infection in a young woman with cystic fibrosis.
Potential benefits
Phage
therapy is the use of bacteriophages to treat bacterial infections.
This could be used as an alternative to antibiotics when bacteria
develop resistance. Superbugs that are immune to multiple types of drugs
are becoming a concern with the more frequent use of antibiotics.
Phages can target these dangerous microbes without harming human cells
due to how specific they are.
Bacteriophage treatment offers a possible alternative to conventional antibiotic treatments for bacterial infection.
It is conceivable that, although bacteria can develop resistance to
phage, the resistance might be easier to overcome than resistance to
antibiotics. Just as bacteria can evolve resistance, viruses can evolve to overcome resistance.
Bacteriophages are very specific, targeting only one or a few strains of bacteria. Traditional antibiotics have a more wide-ranging effect, killing both harmful bacteria and useful bacteria such as those facilitating food digestion.
The species and strain specificity of bacteriophages makes it unlikely
that harmless or useful bacteria will be killed when fighting an
infection.
A few research groups in the West are engineering a broader spectrum phage, and also a variety of forms of MRSA treatments, including impregnated wound dressings, preventative treatment for burn victims, phage-impregnated sutures. Enzybiotics
are a new development at Rockefeller University that create enzymes
from phage. Purified recombinant phage enzymes can be used as separate
antibacterial agents in their own right.
Phage Therapy also has the potential of preventing or treating infectious diseases of corals. This could assist with decline of coral around the world.
Application
Collection
The
simplest method of phage treatment involves collecting local samples of
water likely to contain high quantities of bacteria and bacteriophages,
for example effluent outlets, sewage and other sources. The samples are taken and applied to the bacteria that are to be destroyed which have been cultured on growth medium.
If the bacteria die, as usually happens, the mixture is
centrifuged; the phages collect on the top of the mixture and can be
drawn off.
The phage solutions are then tested to see which ones show growth
suppression effects (lysogeny) or destruction (lysis) of the target
bacteria. The phage showing lysis is then amplified on cultures of the
target bacteria, passed through a filter to remove all but the phages,
then distributed.
Treatment
Phages
are "bacterium-specific" and it is, therefore, necessary in many cases
to take a swab from the patient and culture it prior to treatment.
Occasionally, isolation of therapeutic phages can require a few months
to complete, but clinics generally keep supplies of phage cocktails for
the most common bacterial strains in a geographical area.
Phage cocktails are sold in pharmacies in eastern countries.
The composition of bacteriophagic cocktails has been periodically
modified to add phages effective against emerging pathogenic strains.
Phages in practice are applied orally, topically on infected
wounds or spread onto surfaces, or used during surgical procedures.
Injection is rarely used, avoiding any risks of trace chemical
contaminants that may be present from the bacteria amplification stage,
and recognizing that the immune system naturally fights against viruses
introduced into the bloodstream or lymphatic system.
Individualised phage therapy was successfully used by Robert T. Schooley and others to treat a case of multi-drug-resistant Acinetobacter baumannii in the U.S. in 2015.
Reviews of phage therapy indicate that more clinical and microbiological research is needed to meet current standards.
Clinical trials
Funding
for phage therapy research and clinical trials is generally
insufficient and difficult to obtain, since it is a lengthy and complex
process to patent bacteriophage products. Scientists comment that 'the
biggest hurdle is regulatory', whereas an official view is that
individual phages would need proof individually because it would be too
complicated to do as a combination, with many variables. Due to the
specificity of phages, phage therapy would be most effective with a
cocktail injection, which is generally rejected by the U.S. Food and Drug Administration
(FDA). Researchers and observers predict that for phage therapy to be
successful the FDA must change its regulatory stance on combination drug
cocktails.
Public awareness and education about phage therapy are generally
limited to scientific or independent research rather than mainstream
media.
In 2007 a Phase 1/2 clinical trial was completed at the Royal National Throat, Nose and Ear Hospital, London, for Pseudomonas aeruginosa infections (otitis). Documentation of the Phase-1/Phase-2 study was published in August 2009 in the journal Clinical Otolaryngology.
Phase 1 clinical trials have now been completed in the Southwest
Regional Wound Care Center, Lubbock, Texas for an approved cocktail of
phages against bacteria, including P. aeruginosa, Staphylococcus aureus and Escherichia coli (E. coli). The cocktail of phages for the clinical trials was developed and supplied by Intralytix. PhagoBurn, a phase 1/2 trial of phage therapy against P. aeruginosa wound infection in France and Belgium in 2015–17, was terminated early because the phage therapy was not effective.
Locus Biosciences created a cocktail of three CRISPR modified phages. The study in 2019 of 30 patients will look at the reduction of E. coli in their urinary tracts. Twenty patients will get a phage cocktail, and 10 will get a placebo.
In February 2019, the FDA approved the first clinical trial of intravenously administered phage therapy in the United States.
Administration
Phages can usually be freeze-dried and turned into pills without materially reducing efficiency. Temperature stability up to 55 °C and shelf lives of 14 months have been shown for some types of phages in pill form.
Application in liquid form is possible, stored preferably in refrigerated vials.
Oral administration works better when an antacid is included, as this increases the number of phages surviving passage through the stomach.
Topical administration often involves application to gauzes that are laid on the area to be treated.
IV phage drip therapy was successfully used to treat a patient with MDR Acinetobacter baumannii in Thornton Hospital at UC San Diego in 2017.
In 2019, a Brownsville, MN man with a long-standing bacterial infection in his knee received a phage treatment at the Mayo Clinic which successfully killed the bacteria and avoided planned amputation of his lower leg.
Obstacles
The
high bacterial strain specificity of phage therapy may make it
necessary for clinics to make different cocktails for treatment of the
same infection or disease because the bacterial components of such
diseases may differ from region to region or even person to person. In
addition, this means that 'banks' containing many different phages must
be kept and regularly updated with new phages.
Further, bacteria can evolve different receptors either before or
during treatment. This can prevent phages from completely eradicating
bacteria.
The need for banks of phages makes regulatory testing for safety
harder and more expensive under current rules in most countries. Such a
process would make difficult the large-scale use of phage therapy.
Additionally, patent
issues (specifically on living organisms) may complicate distribution
for pharmaceutical companies wishing to have exclusive rights over their
"invention", which would discourage a commercial corporation from
investing capital in this.
As has been known for at least thirty years, mycobacteria such as Mycobacterium tuberculosis have specific bacteriophages. No lytic phage has yet been discovered for Clostridium difficile, which is responsible for many nosocomial diseases, but some temperate phages (integrated in the genome, also called lysogenic) are known for this species; this opens encouraging avenues but with additional risks as discussed below.
The negative public perception of viruses may also play a role in the reluctance to embrace phage therapy.
Legislation
Approval
of phage therapy for use in humans has not been given in Western
countries with a few exceptions. In the United States, Washington and
Oregon law allows naturopathic physicians to use any therapy that is legal any place in the world on an experimental basis,
and in Texas phages are considered natural substances and can be used
in addition to (but not as a replacement for) traditional therapy (they
have been used routinely in a wound care clinic in Lubbock, TX, since
2006).
In 2013 "the 20th biennial Evergreen International Phage Meeting
... conference drew 170 participants from 35 countries, including
leaders of companies and institutes involved with human phage therapies
from France, Australia, Georgia, Poland and the United States."
Safety
Much of
the difficulty in obtaining regulatory approval is proving to be the
risks of using a self-replicating entity which has the capability to
evolve.
As with antibiotic therapy and other methods of countering bacterial infections, endotoxins are released by the bacteria as they are destroyed within the patient (Herxheimer reaction). This can cause symptoms of fever; in extreme cases toxic shock (a problem also seen with antibiotics) is possible. Janakiraman Ramachandran argues that this complication can be avoided in those types of infection where this reaction is likely to occur by using genetically engineered
bacteriophages which have had their gene responsible for producing
endolysin removed. Without this gene, the host bacterium still dies but
remains intact because the lysis is disabled. On the other hand, this
modification stops the exponential growth of phages, so one administered
phage means one dead bacterial cell. Eventually these dead cells are consumed by the normal house-cleaning duties of the phagocytes, which utilize enzymes to break down the whole bacterium and its contents into harmless proteins, polysaccharides and lipids.
Temperate (or Lysogenic)
bacteriophages are not generally used therapeutically, as this group
can act as a way for bacteria to exchange DNA; this can help spread antibiotic resistance or even, theoretically, make the bacteria pathogenic (see Cholera). Carl Merril claimed that harmless strains of corynebacterium may have been converted into C. diphtheriae that "probably killed a third of all Europeans who came to North America in the seventeenth century". Fortunately, many phages seem to be lytic only with negligible probability of becoming lysogenic.
Other animals
Brigham Young University has been researching the use of phage therapy to treat American foulbrood in honeybees. Phage therapy is also being investigated for potential applications in aquaculture.
Cultural impact
The 1925 novel and 1926 Pulitzer prize winner Arrowsmith used phage therapy as a plot point.
Greg Bear's 2002 novel Vitals features phage therapy, based on Soviet research, used to transfer genetic material.
The 2012 collection of military history essays about the changing
role of women in warfare, "Women in War – from home front to front
line" includes a chapter featuring phage therapy: "Chapter 17: Women who
thawed the Cold War".
Steffanie A. Strathdee's 2019 book The Perfect Predator: An Epidemiologist’s Journey to Save Her Husband from a Deadly Superbug,
co-written with her husband Thomas Patterson, was published by Hachette
Book Group in 2019. It describes Dr Strathdee's ultimately successful
attempt to introduce phage therapy as a life-saving treatment for her
husband, critically ill with a completely antibiotic-resistant Acinetobacter baumannii infection following severe pancreatitis.