From Wikipedia, the free encyclopedia
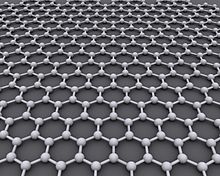
Graphene () is an allotrope of carbon consisting of a single layer of atoms arranged in a two-dimensional honeycomb lattice nanostructure. The name is derived from "graphite" and the suffix -ene, reflecting the fact that the graphite allotrope of carbon contains numerous double bonds.
Each atom in a graphene sheet is connected to its three nearest neighbors by a σ-bond, and contributes one electron to a conduction band that extends over the whole sheet. This is the same type of bonding seen in carbon nanotubes and polycyclic aromatic hydrocarbons, and (partially) in fullerenes and glassy carbon. These conduction bands make graphene a semimetal with unusual electronic properties that are best described by theories for massless relativistic particles.
Charge carriers in graphene show linear, rather than quadratic,
dependence of energy on momentum, and field-effect transistors with
graphene can be made that show bipolar conduction. Charge transport is ballistic over long distances; the material exhibits large quantum oscillations and large and nonlinear diamagnetism.
Graphene conducts heat and electricity very efficiently along its
plane. The material strongly absorbs light of all visible wavelengths,
which accounts for the black color of graphite; yet a single graphene
sheet is nearly transparent because of its extreme thinness. The
material is also about 100 times stronger than would be the strongest
steel of the same thickness.
Photograph
of a suspended graphene membrane in transmitted light. This
one-atom-thick material can be seen with the naked eye because it
absorbs approximately 2.3% of light.
Scientists theorized the potential existence and production of
graphene for decades. It has likely been unknowingly produced in small
quantities for centuries, through the use of pencils and other similar
applications of graphite. It was originally observed in electron microscopes in 1962, but only studied while supported on metal surfaces.
In 2004 the material was rediscovered, isolated and investigated at the University of Manchester, by Andre Geim and Konstantin Novoselov. In 2010 Geim and Novoselov were awarded the Nobel Prize in Physics for their "groundbreaking experiments regarding the two-dimensional material graphene". High-quality graphene proved to be surprisingly easy to isolate.
Graphene has become a valuable and useful nanomaterial
due to its exceptionally high tensile strength, electrical
conductivity, transparency, and being the thinnest two-dimensional
material in the world. The global market for graphene was $9 million in 2012, with most of the demand from research and development in semiconductor, electronics, electric batteries, and composites.
The IUPAC
(International Union for Pure and Applied Chemistry) recommends use of
the name "graphite" for the three-dimensional material, and "graphene"
only when the reactions, structural relations or other properties of
individual layers are discussed.
A narrower definition, of "isolated or free-standing graphene" requires
that the layer be sufficiently isolated from its environment, but would include layers suspended or transferred to silicon dioxide or silicon carbide.
History
Structure of graphite and its intercalation compounds
In 1859 Benjamin Brodie noted the highly lamellar structure of thermally reduced graphite oxide. In 1916, Peter Debije and P. Scherrer determined the structure of graphite by powder X-ray diffraction. The structure was studied in more detail by V. Kohlschütter and P. Haenni in 1918, who also described the properties of graphite oxide paper. Its structure was determined from single-crystal diffraction in 1924.
The theory of graphene was first explored by P. R. Wallace in 1947 as a starting point for understanding the electronic properties of 3D graphite. The emergent massless Dirac equation was first pointed out in 1984 separately by Gordon Walter Semenoff, and by David P. DiVincenzo and Eugene J. Mele. Semenoff emphasized the occurrence in a magnetic field of an electronic Landau level precisely at the Dirac point. This level is responsible for the anomalous integer quantum Hall effect.
Observations of thin graphite layers and related structures
Transmission electron microscopy (TEM) images of thin graphite samples consisting of a few graphene layers were published by G. Ruess and F. Vogt in 1948. Eventually, single layers were also observed directly. Single layers of graphite were also observed by transmission electron microscopy within bulk materials, in particular inside soot obtained by chemical exfoliation.
In 1961–1962, Hanns-Peter Boehm
published a study of extremely thin flakes of graphite, and coined the
term "graphene" for the hypothetical single-layer structure. This paper reports graphitic flakes that give an additional contrast equivalent of down to ~0.4 nm
or 3 atomic layers of amorphous carbon. This was the best possible
resolution for 1960 TEMs. However, neither then nor today is it possible
to argue how many layers were in those flakes. Now we know that the TEM
contrast of graphene most strongly depends on focusing conditions.
For example, it is impossible to distinguish between suspended
monolayer and multilayer graphene by their TEM contrasts, and the only
known way is to analyze the relative intensities of various diffraction
spots. The first reliable TEM observations of monolayers are probably
given in refs. 24 and 26 of Geim and Novoselov's 2007 review.
Starting in the 1970s, C. Oshima and others described single layers of carbon atoms that were grown epitaxially on top of other materials. This "epitaxial graphene" consists of a single-atom-thick hexagonal lattice of sp2-bonded
carbon atoms, as in free-standing graphene. However, there is
significant charge transfer between the two materials, and, in some
cases, hybridization between the d-orbitals
of the substrate atoms and π orbitals of graphene; which significantly
alter the electronic structure compared to that of free-standing
graphene.
The term "graphene" was used again in 1987 to describe single sheets of graphite as a constituent of graphite intercalation compounds, which can be seen as crystalline salts of the intercalant and graphene. It was also used in the descriptions of carbon nanotubes by R. Saito and Mildred and Gene Dresselhaus in 1992, and of polycyclic aromatic hydrocarbons in 2000 by S. Wang and others.
Efforts to make thin films of graphite by mechanical exfoliation started in 1990.
Initial attempts employed exfoliation techniques similar to the drawing
method. Multilayer samples down to 10 nm in thickness were obtained.
In 2002, Robert B. Rutherford and Richard L. Dudman filed for a patent
in the US on a method to produce graphene by repeatedly peeling off
layers from a graphite flake adhered to a substrate, achieving a
graphite thickness of 0.00001 inches (2.5×10−7 metres).
The key to success was high-throughput visual recognition of graphene
on a properly chosen substrate, which provides a small but noticeable
optical contrast.
Another U.S. patent was filed in the same year by Bor Z. Jang and Wen C. Huang for a method to produce graphene based on exfoliation followed by attrition.
Full isolation and characterization
Graphene was properly isolated and characterized in 2004 by Andre Geim and Konstantin Novoselov at the University of Manchester. They pulled graphene layers from graphite with a common adhesive tape in a process called either micromechanical cleavage or the Scotch tape technique. The graphene flakes were then transferred onto thin silicon dioxide (silica) layer on a silicon
plate ("wafer"). The silica electrically isolated the graphene and
weakly interacted with it, providing nearly charge-neutral graphene
layers. The silicon beneath the SiO
2 could be used as a "back gate" electrode to vary the charge density in the graphene over a wide range.
This work resulted in the two winning the Nobel Prize in Physics in 2010 "for groundbreaking experiments regarding the two-dimensional material graphene."
Their publication, and the surprisingly easy preparation method that
they described, sparked a "graphene gold rush". Research expanded and
split off into many different subfields, exploring different exceptional
properties of the material—quantum mechanical, electrical, chemical,
mechanical, optical, magnetic, etc.
Exploring commercial applications
Since
the early 2000s, a number of companies and research laboratories have
been working to develop commercial applications of graphene. In 2014 a National Graphene Institute was established with that purpose at the University of Manchester, with a 60 million GBP initial funding. In North East England two commercial manufacturers, Applied Graphene Materials and Thomas Swan Limited have begun manufacturing. Cambridge Nanosystems is a large-scale graphene powder production facility in East Anglia.
Structure
Bonding
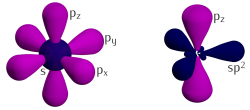
Carbon orbitals 2s, 2px, 2py form the hybrid orbital sp2 with three major lobes at 120°. The remaining orbital, pz, is sticking out of the graphene's plane.
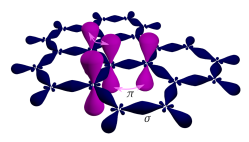
Sigma and pi bonds in graphene. Sigma bonds result from an overlap of sp2 hybrid orbitals, whereas pi bonds emerge from tunneling between the protruding pz orbitals.
Three of the four outer-shell electrons of each atom in a graphene sheet occupy three sp2 hybrid orbitals – a combination of orbitals s, px and py — that are shared with the three nearest atoms, forming σ-bonds. The length of these bonds is about 0.142 nanometers.
The remaining outer-shell electron occupies a pz orbital that is oriented perpendicularly to the plane. These orbitals hybridize together to form two half-filled bands of free-moving electrons, π and π∗, which are responsible for most of graphene's notable electronic properties. Recent quantitative estimates of aromatic stabilization and limiting size derived from the enthalpies of hydrogenation (ΔHhydro) agree well with the literature reports.
Graphene sheets stack to form graphite with an interplanar spacing of 0.335 nm (3.35 Å).
Graphene sheets in solid form usually show evidence in
diffraction for graphite's (002) layering. This is true of some
single-walled nanostructures. However, unlayered graphene with only (hk0) rings has been found in the core of presolar graphite onions. TEM studies show faceting at defects in flat graphene sheets and suggest a role for two-dimensional crystallization from a melt.
Geometry
The hexagonal lattice structure
of isolated, single-layer graphene can be directly seen with
transmission electron microscopy (TEM) of sheets of graphene suspended
between bars of a metallic grid
Some of these images showed a "rippling" of the flat sheet, with
amplitude of about one nanometer. These ripples may be intrinsic to the
material as a result of the instability of two-dimensional crystals, or may originate from the ubiquitous dirt seen in all TEM images of graphene. Photoresist residue, which must be removed to obtain atomic-resolution images, may be the "adsorbates" observed in TEM images, and may explain the observed rippling.
The hexagonal structure is also seen in scanning tunneling microscope (STM) images of graphene supported on silicon dioxide substrates The rippling seen in these images is caused by conformation of graphene to the subtrate's lattice, and is not intrinsic.
Stability
Ab initio calculations show that a graphene sheet is thermodynamically unstable if its size is less than about 20 nm and becomes the most stable fullerene (as within graphite) only for molecules larger than 24,000 atoms.
Properties
Electronic
Electronic
band structure of graphene. Valence and conduction bands meet at the
six vertices of the hexagonal Brillouin zone and form linearly
dispersing Dirac cones.
Graphene is a zero-gap semiconductor, because its conduction and valence bands meet at the Dirac points. The Dirac points are six locations in momentum space, on the edge of the Brillouin zone,
divided into two non-equivalent sets of three points. The two sets are
labeled K and K'. The sets give graphene a valley degeneracy of gv = 2. By contrast, for traditional semiconductors the primary point of interest is generally Γ, where momentum is zero. Four electronic properties separate it from other condensed matter systems.
However, if the in-plane direction is no longer infinite, but
confined, its electronic structure would change. They are referred to as
graphene nanoribbons. If it is "zig-zag", the bandgap would still be zero. If it is "armchair", the bandgap would be non-zero.
Graphene's hexagonal lattice can be regarded as two interleaving
triangular lattices. This perspective was successfully used to calculate
the band structure for a single graphite layer using a tight-binding
approximation.
Electronic spectrum
Electrons propagating through graphene's honeycomb lattice effectively lose their mass, producing quasi-particles that are described by a 2D analogue of the Dirac equation rather than the Schrödinger equation for spin-1⁄2 particles.
Dispersion relation
Electronic band structure and Dirac cones, with effect of
dopingThe cleavage technique led directly to the first observation of the
anomalous quantum Hall effect in graphene in 2005, by Geim's group and
by Philip Kim and Yuanbo Zhang. This effect provided direct evidence of graphene's theoretically predicted Berry's phase of massless Dirac fermions and the first proof of the Dirac fermion nature of electrons. These effects had been observed in bulk graphite by Yakov Kopelevich, Igor A. Luk'yanchuk, and others, in 2003–2004.
When the atoms are placed onto the graphene hexagonal lattice, the overlap between the pz(π) orbitals and the s or the px and py orbitals is zero by symmetry. The pz
electrons forming the π bands in graphene can therefore be treated
independently. Within this π-band approximation, using a conventional tight-binding model, the dispersion relation (restricted to first-nearest-neighbor interactions only) that produces energy of the electrons with wave vector k is

with the nearest-neighbor (π orbitals) hopping energy γ0 ≈ 2.8 eV and the lattice constant a ≈ 2.46 Å. The conduction and valence bands, respectively, correspond to the different signs. With one pz
electron per atom in this model the valence band is fully occupied,
while the conduction band is vacant. The two bands touch at the zone
corners (the K point in the Brillouin zone), where there is a
zero density of states but no band gap. The graphene sheet thus displays
a semimetallic (or zero-gap semiconductor) character, although the same
cannot be said of a graphene sheet rolled into a carbon nanotube,
due to its curvature. Two of the six Dirac points are independent,
while the rest are equivalent by symmetry. In the vicinity of the K-points the energy depends linearly on the wave vector, similar to a relativistic particle. Since an elementary cell of the lattice has a basis of two atoms, the wave function has an effective 2-spinor structure.
As a consequence, at low energies, even neglecting the true spin,
the electrons can be described by an equation that is formally
equivalent to the massless Dirac equation. Hence, the electrons and holes are called Dirac fermions. This pseudo-relativistic description is restricted to the chiral limit, i.e., to vanishing rest mass M0, which leads to interesting additional features:

Here vF ~ 106 m/s (.003 c) is the Fermi velocity in graphene, which replaces the velocity of light in the Dirac theory;  is the vector of the Pauli matrices,
is the vector of the Pauli matrices,  is the two-component wave function of the electrons, and E is their energy.
is the two-component wave function of the electrons, and E is their energy.
The equation describing the electrons' linear dispersion relation is

where the wavevector q is measured from the Brillouin zone vertex K,  ,
and the zero of energy is set to coincide with the Dirac point. The
equation uses a pseudospin matrix formula that describes two sublattices
of the honeycomb lattice.
,
and the zero of energy is set to coincide with the Dirac point. The
equation uses a pseudospin matrix formula that describes two sublattices
of the honeycomb lattice.
Single-atom wave propagation
Electron
waves in graphene propagate within a single-atom layer, making them
sensitive to the proximity of other materials such as high-κ dielectrics, superconductors and ferromagnetics.
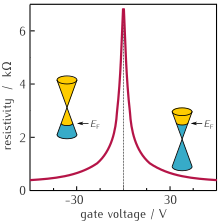
Ambipolar electron and hole transport
When
the gate voltage in a field effect graphene device is changed from
positive to negative, conduction switches from electrons to holes. The
charge carrier concentration is proportional to the applied voltage.
Graphene is neutral at zero gate voltage and resistivity is at its
maximum because of the dearth of charge carriers. The rapid fall of
resistivity when carriers are injected shows their high mobility, here
of the order of 5000 cm2/Vs. n-Si/SiO₂ substrate, T=1K.
Graphene displays remarkable electron mobility at room temperature, with reported values in excess of 15000 cm2⋅V−1⋅s−1. Hole and electron mobilities are nearly the same. The mobility is independent of temperature between 10 K and 100 K, and shows little change even at room temperature (300 K), which implies that the dominant scattering mechanism is defect scattering. Scattering by graphene's acoustic phonons intrinsically limits room temperature mobility in freestanding graphene to 200000 cm2⋅V−1⋅s−1 at a carrier density of 1012 cm−2.
The corresponding resistivity of graphene sheets would be 10−6 Ω⋅cm. This is less than the resistivity of silver, the lowest otherwise known at room temperature. However, on SiO
2
substrates, scattering of electrons by optical phonons of the substrate
is a larger effect than scattering by graphene's own phonons. This
limits mobility to 40000 cm2⋅V−1⋅s−1.
Charge transport has major concerns due to adsorption of
contaminants such as water and oxygen molecules. This leads to
non-repetitive and large hysteresis I-V characteristics. Researchers
must carry out electrical measurements in vacuum. The protection of
graphene surface by a coating with materials such as SiN, PMMA,
h-BN, etc., have been discussed by researchers. In January 2015, the
first stable graphene device operation in air over several weeks was
reported, for graphene whose surface was protected by aluminum oxide. In 2015 lithium-coated graphene exhibited superconductivity, a first for graphene.
Electrical resistance in 40-nanometer-wide nanoribbons
of epitaxial graphene changes in discrete steps. The ribbons'
conductance exceeds predictions by a factor of 10. The ribbons can act
more like optical waveguides or quantum dots,
allowing electrons to flow smoothly along the ribbon edges. In copper,
resistance increases in proportion to length as electrons encounter
impurities.
Transport is dominated by two modes. One is ballistic and
temperature independent, while the other is thermally activated.
Ballistic electrons resemble those in cylindrical carbon nanotubes.
At room temperature, resistance increases abruptly at a particular
length—the ballistic mode at 16 micrometres and the other at 160
nanometres (1% of the former length).
Graphene electrons can cover micrometer distances without scattering, even at room temperature.
Despite zero carrier density near the Dirac points, graphene exhibits a minimum conductivity on the order of  .
The origin of this minimum conductivity is still unclear. However,
rippling of the graphene sheet or ionized impurities in the SiO
.
The origin of this minimum conductivity is still unclear. However,
rippling of the graphene sheet or ionized impurities in the SiO
2 substrate may lead to local puddles of carriers that allow conduction. Several theories suggest that the minimum conductivity should be  ; however, most measurements are of order
; however, most measurements are of order  or greater and depend on impurity concentration.
or greater and depend on impurity concentration.
Near zero carrier density graphene exhibits positive
photoconductivity and negative photoconductivity at high carrier
density. This is governed by the interplay between photoinduced changes
of both the Drude weight and the carrier scattering rate.
Graphene doped with various gaseous species (both acceptors and
donors) can be returned to an undoped state by gentle heating in vacuum. Even for dopant concentrations in excess of 1012 cm−2 carrier mobility exhibits no observable change. Graphene doped with potassium in ultra-high vacuum at low temperature can reduce mobility 20-fold. The mobility reduction is reversible on heating the graphene to remove the potassium.
Due to graphene's two dimensions, charge fractionalization (where
the apparent charge of individual pseudoparticles in low-dimensional
systems is less than a single quantum) is thought to occur. It may therefore be a suitable material for constructing quantum computers using anyonic circuits.
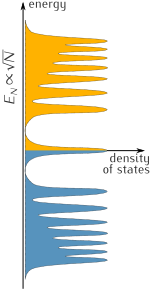
Chiral half-integer quantum Hall effect
Landau levels in graphene appear at energies proportional to √N, in contrast to the standard sequence that goes as N+½.
The quantum Hall effect is a quantum mechanical version of the Hall effect, which is the production of transverse (perpendicular to the main current) conductivity in the presence of a magnetic field. The quantization of the Hall effect  at integer multiples (the "Landau level") of the basic quantity
at integer multiples (the "Landau level") of the basic quantity  (where e is the elementary electric charge and h is Planck's constant). It can usually be observed only in very clean silicon or gallium arsenide solids at temperatures around 3 K and very high magnetic fields.
(where e is the elementary electric charge and h is Planck's constant). It can usually be observed only in very clean silicon or gallium arsenide solids at temperatures around 3 K and very high magnetic fields.
Graphene shows the quantum Hall effect with respect to
conductivity quantization: the effect is unordinary in that the sequence
of steps is shifted by 1/2 with respect to the standard sequence and
with an additional factor of 4. Graphene's Hall conductivity is  , where N is the Landau level and the double valley and double spin degeneracies give the factor of 4. These anomalies are present not only at extremely low temperatures but also at room temperature, i.e. at roughly 20 °C (293 K).
, where N is the Landau level and the double valley and double spin degeneracies give the factor of 4. These anomalies are present not only at extremely low temperatures but also at room temperature, i.e. at roughly 20 °C (293 K).
This behavior is a direct result of graphene's chiral, massless Dirac electrons.
In a magnetic field, their spectrum has a Landau level with energy
precisely at the Dirac point. This level is a consequence of the Atiyah–Singer index theorem and is half-filled in neutral graphene, leading to the "+1/2" in the Hall conductivity. Bilayer graphene also shows the quantum Hall effect, but with only one of the two anomalies (i.e.  ). In the second anomaly, the first plateau at N=0 is absent, indicating that bilayer graphene stays metallic at the neutrality point.
). In the second anomaly, the first plateau at N=0 is absent, indicating that bilayer graphene stays metallic at the neutrality point.
Chiral half-integer quantum Hall effect in graphene. Plateaux in transverse conductivity appear at half integers of 4e²/h.
Unlike normal metals, graphene's longitudinal resistance shows maxima
rather than minima for integral values of the Landau filling factor in
measurements of the Shubnikov–de Haas oscillations, whereby the term integral quantum Hall effect. These oscillations show a phase shift of π, known as Berry's phase.
Berry's phase arises due to chirality or dependence (locking) of the
pseudospin quantum number on momentum of low-energy electrons near the
Dirac points.
The temperature dependence of the oscillations reveals that the
carriers have a non-zero cyclotron mass, despite their zero effective
mass in the Dirac-fermion formalism.
Graphene samples prepared on nickel films, and on both the silicon face and carbon face of silicon carbide, show the anomalous effect directly in electrical measurement. Graphitic layers on the carbon face of silicon carbide show a clear Dirac spectrum in angle-resolved photoemission experiments, and the effect is observed in cyclotron resonance and tunneling experiments.
Strong magnetic fields
In magnetic fields above 10 tesla or so additional plateaus of the Hall conductivity at σxy = νe2/h with ν = 0, ±1, ±4 are observed. A plateau at ν = 3 and the fractional quantum Hall effect at ν = 1⁄3 were also reported.
These observations with ν = 0, ±1, ±3, ±4
indicate that the four-fold degeneracy (two valley and two spin degrees
of freedom) of the Landau energy levels is partially or completely
lifted.
Casimir effect
The Casimir effect
is an interaction between disjoint neutral bodies provoked by the
fluctuations of the electrodynamical vacuum. Mathematically it can be
explained by considering the normal modes of electromagnetic fields,
which explicitly depend on the boundary (or matching) conditions on the
interacting bodies' surfaces. Since graphene/electromagnetic field
interaction is strong for a one-atom-thick material, the Casimir effect
is of growing interest.
Van der Waals force
The Van der Waals force (or dispersion force) is also unusual, obeying an inverse cubic, asymptotic power law in contrast to the usual inverse quartic.
'Massive' electrons
Graphene's
unit cell has two identical carbon atoms and two zero-energy states:
one in which the electron resides on atom A, the other in which the
electron resides on atom B. However, if the two atoms in the unit cell
are not identical, the situation changes. Hunt et al. show that placing hexagonal boron nitride
(h-BN) in contact with graphene can alter the potential felt at atom A
versus atom B enough that the electrons develop a mass and accompanying
band gap of about 30 meV [0.03 Electron Volt(eV)].
The mass can be positive or negative. An arrangement that
slightly raises the energy of an electron on atom A relative to atom B
gives it a positive mass, while an arrangement that raises the energy of
atom B produces a negative electron mass. The two versions behave alike
and are indistinguishable via optical spectroscopy.
An electron traveling from a positive-mass region to a negative-mass
region must cross an intermediate region where its mass once again
becomes zero. This region is gapless and therefore metallic. Metallic
modes bounding semiconducting regions of opposite-sign mass is a
hallmark of a topological phase and display much the same physics as
topological insulators.
If the mass in graphene can be controlled, electrons can be
confined to massless regions by surrounding them with massive regions,
allowing the patterning of quantum dots,
wires, and other mesoscopic structures. It also produces
one-dimensional conductors along the boundary. These wires would be
protected against backscattering and could carry currents without dissipation.
Permittivity
Graphene's permittivity varies with frequency. Over a range from microwave to millimeter wave frequencies it is roughly 3.3. This permittivity, combined with the ability to form both conductors and insulators, means that theoretically, compact capacitors made of graphene could store large amounts of electrical energy.
Optical
Graphene's unique optical properties produce an unexpectedly high opacity for an atomic monolayer in vacuum, absorbing πα ≈ 2.3% of light, from visible to infrared. Here, α is the fine-structure constant. This is a consequence of the "unusual low-energy electronic structure of monolayer graphene that features electron and hole conical bands meeting each other at the Dirac point... [which] is qualitatively different from more common quadratic massive bands."
Based on the Slonczewski–Weiss–McClure (SWMcC) band model of graphite,
the interatomic distance, hopping value and frequency cancel when
optical conductance is calculated using Fresnel equations in the thin-film limit.
Although confirmed experimentally, the measurement is not precise enough to improve on other techniques for determining the fine-structure constant.
Multi-Parametric Surface Plasmon Resonance
was used to characterize both thickness and refractive index of
chemical-vapor-deposition (CVD)-grown graphene films. The measured
refractive index and extinction coefficient values at 670 nm (6.7×10−7 m)
wavelength are 3.135 and 0.897, respectively. The thickness was
determined as 3.7Å from a 0.5mm area, which agrees with 3.35Å reported
for layer-to-layer carbon atom distance of graphite crystals.
The method can be further used also for real-time label-free
interactions of graphene with organic and inorganic substances.
Furthermore, the existence of unidirectional surface plasmons in the
nonreciprocal graphene-based gyrotropic interfaces has been demonstrated
theoretically. By efficiently controlling the chemical potential of
graphene, the unidirectional working frequency can be continuously
tunable from THz to near-infrared and even visible.
Particularly, the unidirectional frequency bandwidth can be 1– 2 orders
of magnitude larger than that in metal under the same magnetic field,
which arises from the superiority of extremely small effective electron
mass in graphene.
Graphene's band gap can be tuned from 0 to 0.25 eV (about 5 micrometre wavelength) by applying voltage to a dual-gate bilayer graphene field-effect transistor (FET) at room temperature. The optical response of graphene nanoribbons is tunable into the terahertz regime by an applied magnetic field. Graphene/graphene oxide systems exhibit electrochromic behavior, allowing tuning of both linear and ultrafast optical properties.
A graphene-based Bragg grating (one-dimensional photonic crystal)
has been fabricated and demonstrated its capability for excitation of
surface electromagnetic waves in the periodic structure by using 633 nm (6.33×10−7 m) He–Ne laser as the light source.
Saturable absorption
Such
unique absorption could become saturated when the input optical
intensity is above a threshold value. This nonlinear optical behavior is
termed saturable absorption
and the threshold value is called the saturation fluence. Graphene can
be saturated readily under strong excitation over the visible to near-infrared region, due to the universal optical absorption and zero band gap. This has relevance for the mode locking of fiber lasers,
where fullband mode locking has been achieved by graphene-based
saturable absorber. Due to this special property, graphene has wide
application in ultrafast photonics. Moreover, the optical response of graphene/graphene oxide layers can be tuned electrically.
Saturable absorption in graphene could occur at the Microwave and
Terahertz band, owing to its wideband optical absorption property. The
microwave saturable absorption in graphene demonstrates the possibility
of graphene microwave and terahertz photonics devices, such as a
microwave saturable absorber, modulator, polarizer, microwave signal
processing and broad-band wireless access networks.
Nonlinear Kerr effect
Under more intensive laser illumination, graphene could also possess a nonlinear phase shift due to the optical nonlinear Kerr effect. Based on a typical open and close aperture z-scan measurement, graphene possesses a giant nonlinear Kerr coefficient of 10−7 cm2⋅W−1, almost nine orders of magnitude larger than that of bulk dielectrics.
This suggests that graphene may be a powerful nonlinear Kerr medium,
with the possibility of observing a variety of nonlinear effects, the
most important of which is the soliton.
Excitonic
First-principle
calculations with quasiparticle corrections and many-body effects are
performed to study the electronic and optical properties of
graphene-based materials. The approach is described as three stages. With GW calculation, the properties of graphene-based materials are accurately investigated, including bulk graphene, nanoribbons, edge and surface functionalized armchair oribbons, hydrogen saturated armchair ribbons, Josephson effect in graphene SNS junctions with single localized defect and armchair ribbon scaling properties.
Spin transport
Graphene is claimed to be an ideal material for spintronics due to its small spin–orbit interaction and the near absence of nuclear magnetic moments in carbon (as well as a weak hyperfine interaction). Electrical spin current injection and detection has been demonstrated up to room temperature. Spin coherence length above 1 micrometre at room temperature was observed, and control of the spin current polarity with an electrical gate was observed at low temperature.
Magnetic properties
Strong magnetic fields
Graphene's quantum Hall effect in magnetic fields above 10 Teslas or so reveals additional interesting features. Additional plateaus of the Hall conductivity at  with
with  are observed. Also, the observation of a plateau at
are observed. Also, the observation of a plateau at  and the fractional quantum Hall effect at
and the fractional quantum Hall effect at  were reported.
were reported.
These observations with  indicate that the four-fold degeneracy (two valley and two spin degrees
of freedom) of the Landau energy levels is partially or completely
lifted. One hypothesis is that the magnetic catalysis of symmetry breaking is responsible for lifting the degeneracy.
indicate that the four-fold degeneracy (two valley and two spin degrees
of freedom) of the Landau energy levels is partially or completely
lifted. One hypothesis is that the magnetic catalysis of symmetry breaking is responsible for lifting the degeneracy.
Spintronic and magnetic properties can be present in graphene simultaneously.
Low-defect graphene nanomeshes manufactured by using a non-lithographic
method exhibit large-amplitude ferromagnetism even at room temperature.
Additionally a spin pumping effect is found for fields applied in
parallel with the planes of few-layer ferromagnetic nanomeshes, while a
magnetoresistance hysteresis loop is observed under perpendicular
fields.
Magnetic substrates
In 2014 researchers magnetized graphene by placing it on an atomically smooth layer of magnetic yttrium iron garnet. The graphene's electronic properties were unaffected. Prior approaches involved doping graphene with other substances. The dopant's presence negatively affected its electronic properties.
Thermal conductivity
Thermal
transport in graphene is an active area of research, which has
attracted attention because of the potential for thermal management
applications. Following predictions for graphene and related carbon nanotubes, early measurements of the thermal conductivity of suspended graphene reported an exceptionally large thermal conductivity up to 5300 W⋅m−1⋅K−1, compared with the thermal conductivity of pyrolytic graphite of approximately 2000 W⋅m−1⋅K−1 at room temperature.
However, later studies primarily on more scalable but more defected
graphene derived by Chemical Vapor Deposition have been unable to
reproduce such high thermal conductivity measurements, producing a wide
range of thermal conductivities between 1500 – 2500 W⋅m−1⋅K−1 for suspended single layer graphene .
The large range in the reported thermal conductivity can be caused by
large measurement uncertainties as well as variations in the graphene
quality and processing conditions.
In addition, it is known that when single-layer graphene is supported on
an amorphous material, the thermal conductivity is reduced to about 500 – 600 W⋅m−1⋅K−1 at room temperature as a result of scattering of graphene lattice waves by the substrate, and can be even lower for few layer graphene encased in amorphous oxide.
Likewise, polymeric residue can contribute to a similar decrease in the
thermal conductivity of suspended graphene to approximately 500 – 600 W⋅m−1⋅K−1 for bilayer graphene.
It has been suggested that the isotopic composition, the ratio of 12C to 13C, has a significant impact on the thermal conductivity. For example, isotopically pure 12C graphene has higher thermal conductivity than either a 50:50 isotope ratio or the naturally occurring 99:1 ratio. It can be shown by using the Wiedemann–Franz law, that the thermal conduction is phonon-dominated. However, for a gated graphene strip, an applied gate bias causing a Fermi energy shift much larger than kBT can cause the electronic contribution to increase and dominate over the phonon contribution at low temperatures. The ballistic thermal conductance of graphene is isotropic.
Potential for this high conductivity can be seen by considering graphite, a 3D version of graphene that has basal plane thermal conductivity of over a 1000 W⋅m−1⋅K−1 (comparable to diamond).
In graphite, the c-axis (out of plane) thermal conductivity is over a
factor of ~100 smaller due to the weak binding forces between basal
planes as well as the larger lattice spacing.
In addition, the ballistic thermal conductance of graphene is shown to
give the lower limit of the ballistic thermal conductances, per unit
circumference, length of carbon nanotubes.
Despite its 2-D nature, graphene has 3 acoustic phonon modes. The two in-plane modes (LA, TA) have a linear dispersion relation, whereas the out of plane mode (ZA) has a quadratic dispersion relation. Due to this, the T2 dependent thermal conductivity contribution of the linear modes is dominated at low temperatures by the T1.5 contribution of the out of plane mode. Some graphene phonon bands display negative Grüneisen parameters.
At low temperatures (where most optical modes with positive Grüneisen
parameters are still not excited) the contribution from the negative
Grüneisen parameters will be dominant and thermal expansion coefficient
(which is directly proportional to Grüneisen parameters) negative. The
lowest negative Grüneisen parameters correspond to the lowest transverse
acoustic ZA modes. Phonon frequencies for such modes increase with the
in-plane lattice parameter
since atoms in the layer upon stretching will be less free to move in
the z direction. This is similar to the behavior of a string, which,
when it is stretched, will have vibrations of smaller amplitude and
higher frequency. This phenomenon, named "membrane effect," was
predicted by Lifshitz in 1952.
Mechanical
The (two-dimensional) density of graphene is 0.763 mg per square meter.
Graphene is the strongest material ever tested, with an intrinsic tensile strength of 130 GPa (19,000,000 psi) (with representative engineering tensile strength ~50-60 GPa for stretching large-area freestanding graphene) and a Young's modulus (stiffness) close to 1 TPa (150,000,000 psi). The Nobel announcement illustrated this by saying that a 1 square meter graphene hammock would support a 4 kg cat but would weigh only as much as one of the cat's whiskers, at 0.77 mg (about 0.001% of the weight of 1 m2 of paper).
Large-angle-bent graphene monolayer has been achieved with
negligible strain, showing mechanical robustness of the two-dimensional
carbon nanostructure. Even with extreme deformation, excellent carrier
mobility in monolayer graphene can be preserved.
The spring constant of suspended graphene sheets has been measured using an atomic force microscope (AFM). Graphene sheets were suspended over SiO
2
cavities where an AFM tip was used to apply a stress to the sheet to
test its mechanical properties. Its spring constant was in the range
1–5 N/m and the stiffness was 0.5 TPa, which differs from that of bulk graphite. These intrinsic properties could lead to applications such as NEMS as pressure sensors and resonators.
Due to its large surface energy and out of plane ductility, flat
graphene sheets are unstable with respect to scrolling, i.e. bending
into a cylindrical shape, which is its lower-energy state.
As is true of all materials, regions of graphene are subject to
thermal and quantum fluctuations in relative displacement. Although the
amplitude of these fluctuations is bounded in 3D structures (even in the
limit of infinite size), the Mermin–Wagner theorem
shows that the amplitude of long-wavelength fluctuations grows
logarithmically with the scale of a 2D structure, and would therefore be
unbounded in structures of infinite size. Local deformation and elastic
strain are negligibly affected by this long-range divergence in
relative displacement. It is believed that a sufficiently large 2D
structure, in the absence of applied lateral tension, will bend and
crumple to form a fluctuating 3D structure. Researchers have observed
ripples in suspended layers of graphene,
and it has been proposed that the ripples are caused by thermal
fluctuations in the material. As a consequence of these dynamical
deformations, it is debatable whether graphene is truly a 2D structure. It has recently been shown that these ripples, if amplified through the introduction of vacancy defects, can impart a negative Poisson's ratio into graphene, resulting in the thinnest auxetic material known so far.
Graphene nanosheets have been incorporated into a Ni matrix
through a plating process to form Ni-graphene composites on a target
substrate. The enhancement in mechanical properties of the composites is
attributed to the high interaction between Ni and graphene and the
prevention of the dislocation sliding in the Ni matrix by the graphene.
Fracture toughness
In 2014, researchers from Rice University and the Georgia Institute of Technology have indicated that despite its strength, graphene is also relatively brittle, with a fracture toughness of about 4 MPa√m. This indicates that imperfect graphene is likely to crack in a brittle manner like ceramic materials,
as opposed to many metallic materials which tend to have fracture
toughnesses in the range of 15–50 MPa√m. Later in 2014, the Rice team
announced that graphene showed a greater ability to distribute force
from an impact than any known material, ten times that of steel per unit
weight. The force was transmitted at 22.2 kilometres per second (13.8 mi/s).
Polycrystalline graphene
Various methods – most notably, chemical vapor deposition
(CVD), as discussed in the section below - have been developed to
produce large-scale graphene needed for device applications. Such
methods often synthesize polycrystalline graphene. The mechanical properties of polycrystalline graphene is affected by the nature of the defects, such as grain-boundaries (GB) and vacancies,
present in the system and the average grain-size. How the mechanical
properties change with such defects have been investigated by
researchers, theoretically and experimentally.
Graphene grain boundaries typically contain heptagon-pentagon
pairs. The arrangement of such defects depends on whether the GB is in
zig-zag or armchair direction. It further depends on the tilt-angle of
the GB.
In 2010, researchers from Brown University computationally predicted
that as the tilt-angle increases, the grain boundary strength also
increases. They showed that the weakest link in the grain boundary is at
the critical bonds of the heptagon rings. As the grain boundary angle
increases, the strain in these heptagon rings decreases, causing the
grain-boundary to be stronger than lower-angle GBs. They proposed that,
in fact, for sufficiently large angle GB, the strength of the GB is
similar to pristine graphene.
In 2012, it was further shown that the strength can increase or
decrease, depending on the detailed arrangements of the defects.
These predictions have since been supported by experimental evidences.
In a 2013 study led by James Hone's group, researchers probed the
elastic stiffness and strength of CVD-grown graphene by combining nano-indentation and high-resolution TEM. They found that the elastic stiffness is identical and strength is only slightly lower than those in pristine graphene. In the same year, researchers from UC Berkeley and UCLA probed bi-crystalline graphene with TEM and AFM. They found that the strength of grain-boundaries indeed tend to increase with the tilt angle.
While the presence of vacancies is not only prevalent in
polycrystalline graphene, vacancies can have significant effects on the
strength of graphene. The general consensus is that the strength
decreases along with increasing densities of vacancies. In fact, various
studies have shown that for graphene with sufficiently low density of
vacancies, the strength does not vary significantly from that of
pristine graphene. On the other hand, high density of vacancies can
severely reduce the strength of graphene.
Compared to the fairly well-understood nature of the effect that
grain boundary and vacancies have on the mechanical properties of
graphene, there is no clear consensus on the general effect that the
average grain size has on the strength of polycrystalline graphene. In fact, three notable theoretical/computational studies on this topic have led to three different conclusions. First, in 2012, Kotakoski and Myer studied the mechanical properties of
polycrystalline graphene with "realistic atomistic model", using molecular-dynamics (MD) simulation. To emulate the growth mechanism of CVD, they first randomly selected nucleation
sites that are at least 5A (arbitrarily chosen) apart from other sites.
Polycrystalline graphene was generated from these nucleation sites and
was subsequently annealed at 3000K, then quenched. Based on this model,
they found that cracks are initiated at grain-boundary junctions, but
the grain size does not significantly affect the strength.
Second, in 2013, Z. Song et al. used MD simulations to study the
mechanical properties of polycrystalline graphene with uniform-sized
hexagon-shaped grains. The hexagon grains were oriented in various
lattice directions and the GBs consisted of only heptagon, pentagon, and
hexagonal carbon rings. The motivation behind such model was that
similar systems had been experimentally observed in graphene flakes
grown on the surface of liquid copper. While they also noted that crack
is typically initiated at the triple junctions, they found that as the
grain size decreases, the yield strength of graphene increases. Based on
this finding, they proposed that polycrystalline follows pseudo Hall-Petch relationship.
Third, in 2013, Z. D. Sha et al. studied the effect of grain size on
the properties of polycrystalline graphene, by modelling the grain
patches using Voronoi construction.
The GBs in this model consisted of heptagon, pentagon, and hexagon, as
well as squares, octagons, and vacancies. Through MD simulation,
contrary to the fore-mentioned study, they found inverse Hall-Petch
relationship, where the strength of graphene increases as the grain size
increases. Experimental observations and other theoretical predictions also gave differing conclusions, similar to the three given above.
Such discrepancies show the complexity of the effects that grain size,
arrangements of defects, and the nature of defects have on the
mechanical properties of polycrystalline graphene.
Chemical
Graphene has a theoretical specific surface area (SSA) of 2630 m2/g. This is much larger than that reported to date for carbon black (typically smaller than 900 m2/g) or for carbon nanotubes (CNTs), from ≈100 to 1000 m2/g and is similar to activated carbon.
Graphene is the only form of carbon (or solid material) in which every
atom is available for chemical reaction from two sides (due to the 2D
structure). Atoms at the edges of a graphene sheet have special chemical
reactivity. Graphene has the highest ratio of edge atoms of any allotrope. Defects within a sheet increase its chemical reactivity. The onset temperature of reaction between the basal plane of single-layer graphene and oxygen gas is below 260 °C (530 K). Graphene burns at very low temperature (e.g., 350 °C (620 K)).
Graphene is commonly modified with oxygen- and nitrogen-containing
functional groups and analyzed by infrared spectroscopy and X-ray
photoelectron spectroscopy. However, determination of structures of
graphene with oxygen- and nitrogen- functional groups requires the structures to be well controlled.
In 2013, Stanford University physicists reported that single-layer graphene is a hundred times more chemically reactive than thicker multilayer sheets.
Graphene can self-repair holes in its sheets, when exposed to molecules containing carbon, such as hydrocarbons. Bombarded with pure carbon atoms, the atoms perfectly align into hexagons, completely filling the holes.
Biological
Despite
the promising results in different cell studies and proof of concept
studies, there is still incomplete understanding of the full
biocompatibility of graphene based materials.
Different cell lines react differently when exposed to graphene, and it
has been shown that the lateral size of the graphene flakes, the form
and surface chemistry can elicit different biological responses on the
same cell line.
There are indications that graphene has promise as a useful
material for interacting with neural cells; studies on cultured neural
cells show limited success.
Graphene also has some utility in osteogenics.
Researchers at the Graphene Research Centre at the National University
of Singapore (NUS) discovered in 2011 the ability of graphene to
accelerate the osteogenic differentiation of human Mesenchymal Stem Cells without the use of biochemical inducers.
Graphene can be used in biosensors; in 2015 researchers
demonstrated that a graphene-based sensor be can used to detect a cancer
risk biomarker. In particular, by using epitaxial graphene on silicon
carbide, they were repeatably able to detect 8-hydroxydeoxyguanosine
(8-OHdG), a DNA damage biomarker.
Support substrate
The
electronics property of graphene can be significantly influenced by the
supporting substrate. Studies of graphene monolayers on clean and
hydrogen(H)-passivated silicon (100) (Si(100)/H) surfaces have been
performed.
The Si(100)/H surface does not perturb the electronic properties of
graphene, whereas the interaction between the clean Si(100) surface and
graphene changes the electronic states of graphene significantly. This
effect results from the covalent bonding between C and surface Si atoms,
modifying the π-orbital network of the graphene layer. The local
density of states shows that the bonded C and Si surface states are
highly disturbed near the Fermi energy.
Forms
Monolayer sheets
In 2013 a group of Polish scientists presented a production unit that allows the manufacture of continuous monolayer sheets. The process is based on graphene growth on a liquid metal matrix. The product of this process was called High Strength Metallurgical Graphene.
In a new study published in Nature, the researchers have used a single
layer graphene electrode and a novel surface sensitive non-linear
spectroscopy technique to investigate the top-most water layer at the
electrochemically charged surface. They found that the interfacial water
response to applied electric field is asymmetric with respect to the
nature of the applied field.
Bilayer graphene
Bilayer graphene displays the anomalous quantum Hall effect, a tunable band gap and potential for excitonic condensation –making it a promising candidate for optoelectronic and nanoelectronic applications. Bilayer graphene typically can be found either in twisted
configurations where the two layers are rotated relative to each other
or graphitic Bernal stacked configurations where half the atoms in one
layer lie atop half the atoms in the other. Stacking order and orientation govern the optical and electronic properties of bilayer graphene.
One way to synthesize bilayer graphene is via chemical vapor deposition, which can produce large bilayer regions that almost exclusively conform to a Bernal stack geometry.
It has been shown that the two graphene layers can withstand important strain or doping mismatch which ultimately should lead to their exfoliation.
Turbostratic graphene
Turbostratic
graphene exhibits weak interlayer coupling, and the spacing is
increased with respect to Bernal-stacked multilayer graphene. Rotational
misalignment preserves the 2D electronic structure, as confirmed by
Raman spectroscopy. The D peak is very weak, whereas the 2D and G peaks
remain prominent. A rather peculiar feature is that the I2D/IG ratio can exceed 10. However, most importantly, the M peak, which originates from AB stacking, is absent, whereas the TS1 and TS2 modes are visible in the Raman spectrum.
The material is formed through conversion of non-graphenic carbon into
graphenic carbon without providing sufficient energy to allow for the
reorganization through annealing of adjacent graphene layers into
crystalline graphitic structures.
Graphene superlattices
Periodically
stacked graphene and its insulating isomorph provide a fascinating
structural element in implementing highly functional superlattices at
the atomic scale, which offers possibilities in designing nanoelectronic
and photonic devices. Various types of superlattices can be obtained by
stacking graphene and its related forms.
The energy band in layer-stacked superlattices is found to be more
sensitive to the barrier width than that in conventional III–V
semiconductor superlattices. When adding more than one atomic layer to
the barrier in each period, the coupling of electronic wavefunctions in
neighboring potential wells can be significantly reduced, which leads to
the degeneration of continuous subbands into quantized energy levels.
When varying the well width, the energy levels in the potential wells
along the L-M direction behave distinctly from those along the K-H
direction.
A superlattice corresponds to a periodic or quasi-periodic
arrangement of different materials, and can be described by a
superlattice period which confers a new translational symmetry to the
system, impacting their phonon dispersions and subsequently their
thermal transport properties.
Recently, uniform monolayer graphene-hBN structures have been
successfully synthesized via lithography patterning coupled with
chemical vapor deposition (CVD).
Furthermore, superlattices of graphene-hBN are ideal model systems for
the realization and understanding of coherent (wave-like) and incoherent
(particle-like) phonon thermal transport.
Graphene nanoribbons
Names for graphene edge topologies
GNR
Electronic band structure of graphene strips of varying widths in
zig-zag orientation. Tight-binding calculations show that they are all
metallic.
GNR
Electronic band structure of graphene strips of various widths in the
armchair orientation. Tight-binding calculations show that they are
semiconducting or metallic depending on width (chirality).
Graphene nanoribbons
("nanostripes" in the "zig-zag"/"zigzag" orientation), at low
temperatures, show spin-polarized metallic edge currents, which also
suggests applications in the new field of spintronics. (In the "armchair" orientation, the edges behave like semiconductors.)
Graphene quantum dots
A graphene quantum dot
(GQD) is a graphene fragment with size less than 100 nm. The
properties of GQDs are different from 'bulk' graphene due to the quantum
confinement effects which only becomes apparent when size is smaller
than 100 nm.
Graphene oxide
Graphene oxide is usually produced through chemical exfoliation of
graphite. A particularly popular technique is the improved Hummer's
method.
Using paper-making techniques on dispersed, oxidized and chemically
processed graphite in water, the monolayer flakes form a single sheet
and create strong bonds. These sheets, called graphene oxide paper, have a measured tensile modulus of 32 GPa.
The chemical property of graphite oxide is related to the functional
groups attached to graphene sheets. These can change the polymerization
pathway and similar chemical processes. Graphene oxide flakes in polymers display enhanced photo-conducting properties.
Graphene is normally hydrophobic and impermeable to all gases and
liquids (vacuum-tight). However, when formed into graphene oxide-based
capillary membrane, both liquid water and water vapor flow through as
quickly as if the membrane was not present.
Chemical modification
Photograph
of single-layer graphene oxide undergoing high temperature chemical
treatment, resulting in sheet folding and loss of carboxylic
functionality, or through room temperature carbodiimide treatment,
collapsing into star-like clusters.
Soluble fragments of graphene can be prepared in the laboratory
through chemical modification of graphite. First, microcrystalline
graphite is treated with an acidic mixture of sulfuric acid and nitric acid. A series of oxidation and exfoliation steps produce small graphene plates with carboxyl groups at their edges. These are converted to acid chloride groups by treatment with thionyl chloride; next, they are converted to the corresponding graphene amide via treatment with octadecylamine. The resulting material (circular graphene layers of 5.3 Å or 5.3×10−10 m thickness) is soluble in tetrahydrofuran, tetrachloromethane and dichloroethane.
Refluxing single-layer graphene oxide (SLGO) in solvents
leads to size reduction and folding of individual sheets as well as
loss of carboxylic group functionality, by up to 20%, indicating thermal
instabilities of SLGO sheets dependent on their preparation
methodology. When using thionyl chloride, acyl chloride groups result, which can then form aliphatic and aromatic amides with a reactivity conversion of around 70–80%.
Boehm
titration results for various chemical reactions of single-layer
graphene oxide, which reveal reactivity of the carboxylic groups and the
resultant stability of the SLGO sheets after treatment.
Hydrazine reflux is commonly used for reducing SLGO to SLG(R), but titrations
show that only around 20–30% of the carboxylic groups are lost, leaving
a significant number available for chemical attachment. Analysis of
SLG(R) generated by this route reveals that the system is unstable and
using a room temperature stirring with HCl (< 1.0 M) leads to around
60% loss of COOH functionality. Room temperature treatment of SLGO with carbodiimides
leads to the collapse of the individual sheets into star-like clusters
that exhibited poor subsequent reactivity with amines (c. 3–5%
conversion of the intermediate to the final amide).
It is apparent that conventional chemical treatment of carboxylic
groups on SLGO generates morphological changes of individual sheets that
leads to a reduction in chemical reactivity, which may potentially
limit their use in composite synthesis. Therefore, chemical reactions
types have been explored. SLGO has also been grafted with polyallylamine, cross-linked through epoxy
groups. When filtered into graphene oxide paper, these composites
exhibit increased stiffness and strength relative to unmodified graphene
oxide paper.
Full hydrogenation from both sides of graphene sheet results in graphane, but partial hydrogenation leads to hydrogenated graphene. Similarly, both-side fluorination of graphene (or chemical and mechanical exfoliation of graphite fluoride) leads to fluorographene (graphene fluoride), while partial fluorination (generally halogenation) provides fluorinated (halogenated) graphene.
Graphene ligand/complex
Graphene can be a ligand to coordinate metals and metal ions by introducing functional groups. Structures of graphene ligands are similar to e.g. metal-porphyrin complex, metal-phthalocyanine complex, and metal-phenanthroline complex. Copper and nickel ions can be coordinated with graphene ligands.
Graphene fiber
In
2011, researchers reported a novel yet simple approach to fabricate
graphene fibers from chemical vapor deposition grown graphene films.
The method was scalable and controllable, delivering tunable morphology
and pore structure by controlling the evaporation of solvents with
suitable surface tension. Flexible all-solid-state supercapacitors based
on this graphene fibers were demonstrated in 2013.
In 2015 intercalating small graphene fragments into the gaps
formed by larger, coiled graphene sheets, after annealing provided
pathways for conduction, while the fragments helped reinforce the
fibers.
The resulting fibers offered better thermal and electrical conductivity
and mechanical strength. Thermal conductivity reached 1,290 W/m/K (1,290 watts per metre per kelvin), while tensile strength reached 1,080 MPa (157,000 psi).
In 2016, Kilometer-scale continuous graphene fibers with
outstanding mechanical properties and excellent electrical conductivity
are produced by high-throughput wet-spinning of graphene oxide liquid
crystals followed by graphitization through a full-scale synergetic
defect-engineering strategy.
The graphene fibers with superior performances promise wide
applications in functional textiles, lightweight motors, microelectronic
devices, etc.
Tsinghua University in Beijing, led by Wei Fei of the Department
of Chemical Engineering, claims to be able to create a carbon nanotube
fibre which has a tensile strength of 80 GPa (12,000,000 psi).
3D graphene
In 2013, a three-dimensional honeycomb of hexagonally arranged carbon was termed 3D graphene, and self-supporting 3D graphene was also produced.
3D structures of graphene can be fabricated by using either CVD or
solution based methods. A 2016 review by Khurram and Xu et al. provided a
summary of then-state-of-the-art techniques for fabrication of the 3D
structure of graphene and other related two-dimensional materials.
In 2013, researchers at Stony Brook University reported a novel
radical-initiated crosslinking method to fabricate porous 3D
free-standing architectures of graphene and carbon nanotubes using
nanomaterials as building blocks without any polymer matrix as support.
These 3D graphene (all-carbon) scaffolds/foams have applications in
several fields such as energy storage, filtration, thermal management
and biomedical devices and implants.
Box-shaped graphene (BSG) nanostructure appearing after mechanical cleavage of pyrolytic graphite was reported in 2016.[225]
The discovered nanostructure is a multilayer system of parallel hollow
nanochannels located along the surface and having quadrangular
cross-section. The thickness of the channel walls is approximately equal
to 1 nm. Potential fields of BSG application include: ultra-sensitive detectors, high-performance catalytic cells, nanochannels for DNA sequencing and manipulation, high-performance heat sinking surfaces, rechargeable batteries of enhanced performance, nanomechanical resonators, electron multiplication channels in emission nanoelectronic devices, high-capacity sorbents for safe hydrogen storage.
Three dimensional bilayer graphene has also been reported.
Pillared graphene
Pillared graphene is a hybrid carbon, structure consisting of an
oriented array of carbon nanotubes connected at each end to a sheet of
graphene. It was first described theoretically by George Froudakis and
colleagues of the University of Crete in Greece in 2008. Pillared
graphene has not yet been synthesised in the laboratory, but it has been
suggested that it may have useful electronic properties, or as a
hydrogen storage material.
Reinforced graphene
Graphene reinforced with embedded carbon nanotube reinforcing bars ("rebar") is easier to manipulate, while improving the electrical and mechanical qualities of both materials.
Functionalized single- or multiwalled carbon nanotubes are
spin-coated on copper foils and then heated and cooled, using the
nanotubes themselves as the carbon source. Under heating, the functional
carbon groups decompose into graphene, while the nanotubes partially split and form in-plane covalent bonds with the graphene, adding strength. π–π stacking
domains add more strength. The nanotubes can overlap, making the
material a better conductor than standard CVD-grown graphene. The
nanotubes effectively bridge the grain boundaries
found in conventional graphene. The technique eliminates the traces of
substrate on which later-separated sheets were deposited using epitaxy.
Stacks of a few layers have been proposed as a cost-effective and physically flexible replacement for indium tin oxide (ITO) used in displays and photovoltaic cells.
Moulded graphene
In 2015, researchers from the University of Illinois at Urbana-Champaign (UIUC) developed a new approach for forming 3D shapes from flat, 2D sheets of graphene.
A film of graphene that had been soaked in solvent to make it swell and
become malleable was overlaid on an underlying substrate "former". The
solvent evaporated over time, leaving behind a layer of graphene that
had taken on the shape of the underlying structure. In this way they
were able to produce a range of relatively intricate micro-structured
shapes.
Features vary from 3.5 to 50 μm. Pure graphene and gold-decorated
graphene were each successfully integrated with the substrate.
Graphene aerogel
An aerogel
made of graphene layers separated by carbon nanotubes was measured at
0.16 milligrams per cubic centimeter. A solution of graphene and carbon
nanotubes in a mold is freeze dried to dehydrate the solution, leaving
the aerogel. The material has superior elasticity and absorption. It can
recover completely after more than 90% compression, and absorb up to
900 times its weight in oil, at a rate of 68.8 grams per second.
Graphene nanocoil
In
2015 a coiled form of graphene was discovered in graphitic carbon
(coal). The spiraling effect is produced by defects in the material's
hexagonal grid that causes it to spiral along its edge, mimicking a Riemann surface,
with the graphene surface approximately perpendicular to the axis. When
voltage is applied to such a coil, current flows around the spiral,
producing a magnetic field. The phenomenon applies to spirals with
either zigzag or armchair patterns, although with different current
distributions. Computer simulations indicated that a conventional spiral
inductor of 205 microns in diameter could be matched by a nanocoil just
70 nanometers wide, with a field strength reaching as much as 1 tesla.
The nano-solenoids analyzed through computer models at Rice
should be capable of producing powerful magnetic fields of about
1 tesla, about the same as the coils found in typical loudspeakers,
according to Yakobson and his team – and about the same field strength
as some MRI machines. They found the magnetic field would be strongest
in the hollow, nanometer-wide cavity at the spiral's center.
A solenoid
made with such a coil behaves as a quantum conductor whose current
distribution between the core and exterior varies with applied voltage,
resulting in nonlinear inductance.
Crumpled graphene
In 2016, Brown University
introduced a method for 'crumpling' graphene, adding wrinkles to the
material on a nanoscale. This was achieved by depositing layers of
graphene oxide onto a shrink film, then shrunken, with the film
dissolved before being shrunken again on another sheet of film. The
crumpled graphene became superhydrophobic, and, when used as a battery electrode, the material was shown to have as much as a 400% increase in electrochemical current density.
Production
A rapidly increasing list of production techniques have been developed to enable graphene's use in commercial applications.
Isolated 2D crystals cannot be grown via chemical synthesis beyond small sizes even in principle, because the rapid growth of phonon
density with increasing lateral size forces 2D crystallites to bend
into the third dimension. In all cases, graphene must bond to a
substrate to retain its two-dimensional shape.
Small graphene structures, such as graphene quantum dots and
nanoribbons, can be produced by "bottom up" methods that assemble the
lattice from organic molecule monomers (e. g. citric acid, glucose).
"Top down" methods, on the other hand, cut bulk graphite and graphene
materials with strong chemicals (e. g. mixed acids).
Mechanical
Mechanical exfoliation
Geim and Novoselov initially used adhesive tape
to pull graphene sheets away from graphite. Achieving single layers
typically requires multiple exfoliation steps. After exfoliation the
flakes are deposited on a silicon wafer. Crystallites larger than 1 mm
and visible to the naked eye can be obtained.
As of 2014, exfoliation produced graphene with the lowest number of defects and highest electron mobility.
Alternatively a sharp single-crystal diamond wedge penetrates onto the graphite source to cleave layers.
In 2014 defect-free, unoxidized graphene-containing liquids were
made from graphite using mixers that produce local shear rates greater
than 10×104.
Shear exfoliation is another method which by using rotor-stator
mixer the scalable production of the defect-free Graphene has become
possible It has been shown that, as turbulence is not necessary for mechanical exfoliation, low speed ball milling is shown to be effective in the production of High-Yield and water-soluble graphene.
Ultrasonic exfoliation
Dispersing graphite in a liquid medium can produce graphene by sonication followed by centrifugation, producing concentrations 2.1 mg/ml in N-methylpyrrolidone. Using a suitable ionic liquid as the dispersing liquid medium produced concentrations of 5.33 mg/ml. Restacking is an issue with this technique.
Adding a surfactant
to a solvent prior to sonication prevents restacking by adsorbing to
the graphene's surface. This produces a higher graphene concentration,
but removing the surfactant requires chemical treatments.
Sonicating graphite at the interface of two immiscible liquids, most notably heptane
and water, produced macro-scale graphene films. The graphene sheets are
adsorbed to the high energy interface between the materials and are
kept from restacking. The sheets are up to about 95% transparent and
conductive.
With definite cleavage parameters, the box-shaped graphene (BSG) nanostructure can be prepared on graphite crystal.
Splitting monolayer carbon
Nanotube slicing
Graphene can be created by opening carbon nanotubes by cutting or etching. In one such method multi-walled carbon nanotubes are cut open in solution by action of potassium permanganate and sulfuric acid.
In 2014, carbon nanotube-reinforced graphene was made via spin coating and annealing functionalized carbon nanotubes.
Fullerene splitting
Another approach sprays buckyballs
at supersonic speeds onto a substrate. The balls cracked open upon
impact, and the resulting unzipped cages then bond together to form a
graphene film.
Chemical
Graphite oxide reduction
P. Boehm reported producing monolayer flakes of reduced graphene oxide in 1962. Rapid heating of graphite oxide and exfoliation yields highly dispersed carbon powder with a few percent of graphene flakes.
Another method is reduction of graphite oxide monolayer films, e.g. by hydrazine with annealing in argon/hydrogen with an almost intact carbon framework that allows efficient removal of functional groups. Measured charge carrier mobility exceeded 1000 cm/Vs (10m/Vs).
Burning a graphite oxide coated DVD
produced a conductive graphene film (1738 siemens per meter) and
specific surface area (1520 square meters per gram) that was highly
resistant and malleable.
A dispersed reduced graphene oxide suspension was synthesized in
water by a hydrothermal dehydration method without using any surfactant.
The approach is facile, industrially applicable, environmentally
friendly and cost effective. Viscosity measurements confirmed that the
graphene colloidal suspension (Graphene nanofluid) exhibit Newtonian
behavior, with the viscosity showing close resemblance to that of water.
Molten salts
Graphite particles can be corroded in molten salts to form a variety of carbon nanostructures including graphene.
Hydrogen cations, dissolved in molten lithium chloride, can be
discharged on cathodically polarized graphite rods, which then
intercalate, peeling graphene sheets. The graphene nanosheets produced
displayed a single-crystalline structure with a lateral size of several
hundred nanometers and a high degree of crystallinity and thermal
stability.
Electrochemical synthesis
Electrochemical
synthesis can exfoliate graphene. Varying a pulsed voltage controls
thickness, flake area, number of defects and affects its properties. The
process begins by bathing the graphite in a solvent for intercalation.
The process can be tracked by monitoring the solution's transparency
with an LED and photodiode.
Hydrothermal self-assembly
Graphene has been prepared by using a sugar (e.g. glucose, sugar, fructose,
etc.) This substrate-free "bottom-up" synthesis is safer, simpler and
more environmentally friendly than exfoliation. The method can control
thickness, ranging from monolayer to multilayers, which is known as
"Tang-Lau Method".
Sodium ethoxide pyrolysis
Gram-quantities were produced by the reaction of ethanol with sodium metal, followed by pyrolysis and washing with water.
Microwave-assisted oxidation
In 2012, microwave energy was reported to directly synthesize graphene in one step.
This approach avoids use of potassium permanganate in the reaction
mixture. It was also reported that by microwave radiation assistance,
graphene oxide with or without holes can be synthesized by controlling
microwave time. Microwave heating can dramatically shorten the reaction time from days to seconds.
Graphene can also be made by microwave assisted hydrothermal pyrolysis.
Thermal decomposition of silicon carbide
Heating silicon carbide (SiC) to high temperatures (1100 °C) under low pressures (c. 10−6 torr, or 10−4 Pa) reduces it to graphene.
Chemical vapor deposition
Epitaxy
Epitaxial graphene growth on silicon carbide is wafer-scale technique to produce graphene. Epitaxial graphene may be coupled to surfaces weakly enough (by Van der Waals forces) to retain the two dimensional electronic band structure of isolated graphene.
A normal silicon wafer coated with a layer of germanium (Ge) dipped in dilute hydrofluoric acid strips the naturally forming germanium oxide groups, creating hydrogen-terminated germanium. CVD can coat that with graphene.
The direct synthesis of graphene on insulator TiO2 with high-dielectric-constant (high-κ). A two-step CVD process is shown to grow graphene directly on TiO2 crystals or exfoliated TiO2 nanosheets without using any metal catalyst.
Metal substrates
CVD graphene can be grown on metal substrates including ruthenium, iridium, nickel and copper.
Roll-to-roll
In
2014 a two-step roll-to-roll manufacturing process was announced. The
first roll-to-roll step produces the graphene via chemical vapor
deposition. The second step binds the graphene to a substrate.
Large-area Raman mapping of CVD graphene on deposited Cu thin film on 150 mm SiO2/Si wafers reveals >95% monolayer continuity and an average value of ∼2.62 for I2D/IG. The scale bar is 200 μm.
Cold wall
Growing
graphene in an industrial resistive-heating cold wall CVD system was
claimed to produce graphene 100 times faster than conventional CVD
systems, cut costs by 99% and produce material with enhanced electronic
qualities.
Wafer scale CVD graphene
CVD graphene is scalable and has been grown on deposited Cu thin film catalyst on 100 to 300 mm standard Si/SiO2 wafers
on an Axitron Black Magic system. Monolayer graphene coverage of
>95% is achieved on 100 to 300 mm wafer substrates with negligible
defects, confirmed by extensive Raman mapping.
Carbon dioxide reduction
A highly exothermic reaction combusts magnesium in an oxidation–reduction reaction with carbon dioxide, producing carbon nanoparticles including graphene and fullerenes.
Supersonic spray
Supersonic acceleration of droplets through a Laval nozzle
was used to deposit reduced graphene-oxide on a substrate. The energy
of the impact rearranges that carbon atoms into flawless graphene.
Laser
In 2014, a CO
2 infrared laser
was used to produce patterned porous three-dimensional laser-induced
graphene (LIG) film networks from commercial polymer films. The
resulting material exhibits high electrical conductivity and surface
area. The laser induction process is compatible with roll-to-roll
manufacturing processes. A similar material, laser-induced graphene fibers (LIGF), was reported in 2018.
Flash Joule heating
In
2019, flash Joule heating (transient high-temperature electrothermal
heating) was discovered to be a method to synthesize turbostratic
graphene in bulk powder form. The method involves electrothermally
converting various carbon sources, such as carbon black, coal, and food
waste into micron-scale flakes of graphene. More recent works demonstrated the use of mixed plastic waste, waste rubber tires, and pyrolysis ash as carbon feedstocks.
The graphenization process is kinetically controlled, and the energy
dose is chosen to preserve the carbon in its graphenic state (excessive
energy input leads to subsequent graphitization through annealing).
Ion implantation
Accelerating carbon ions inside an electrical field into a semiconductor made of thin nickel films on a substrate of SiO2/Si,
creates a wafer-scale (4 inches (100 mm)) wrinkle/tear/residue-free
graphene layer at a relatively low temperature of 500 °C.
CMOS-compatible graphene
Integration of graphene in the widely employed CMOS fabrication process demands its transfer-free direct synthesis on dielectric substrates at temperatures below 500 °C. At the IEDM 2018, researchers from University of California, Santa Barbara, demonstrated a novel CMOS-compatible graphene synthesis process at 300 °C suitable for back-end-of-line (BEOL) applications. The process involves pressure-assisted solid-state diffusion of carbon through a thin-film of metal catalyst. The synthesized large-area graphene films were shown to exhibit high-quality (via Raman characterization) and similar resistivity values when compared with high-temperature CVD synthesized graphene films of same cross-section down to widths of 20 nm.
Simulation
In
addition to experimental investigation of graphene and graphene-based
devices, their numerical modeling and simulation have been an important
research topic. The Kubo formula provides an analytic expression for the
graphene's conductivity and shows that it is a function of several
physical parameters including wavelength, temperature, and chemical
potential.
Moreover, a surface conductivity model, which describes graphene as an
infinitesimally thin (two sided) sheet with a local and isotropic
conductivity, has been proposed. This model permits derivation of
analytical expressions for the electromagnetic field in the presence of a
graphene sheet in terms of a dyadic Green function (represented using
Sommerfeld integrals) and exciting electric current.
Even though these analytical models and methods can provide results for
several canonical problems for benchmarking purposes, many practical
problems involving graphene, such as design of arbitrarily shaped
electromagnetic devices, are analytically intractable. With the recent
advances in the field of computational electromagnetics (CEM), various
accurate and efficient numerical methods have become available for
analysis of electromagnetic field/wave interactions on graphene sheets
and/or graphene-based devices. A comprehensive summary of computational
tools developed for analyzing graphene-based devices/systems is
proposed.
Graphene analogs
Graphene analogs
(also referred to as "artificial graphene") are two-dimensional systems
which exhibit similar properties to graphene. Graphene analogs are
studied intensively since the discovery of graphene in 2004. People try
to develop systems in which the physics is easier to observe and to
manipulate than in graphene. In those systems, electrons are not always
the particles which are used. They might be optical photons, microwave photons, plasmons, microcavity polaritons, or even atoms.
Also, the honeycomb structure in which those particles evolve can be of
a different nature than carbon atoms in graphene. It can be,
respectively, a photonic crystal, an array of metallic rods, metallic nanoparticles, a lattice of coupled microcavities, or an optical lattice.
Applications
(a)
The typical structure of a touch sensor in a touch panel. (Image
courtesy of Synaptics, Incorporated.) (b) An actual example of 2D Carbon
Graphene Material Co.,Ltd's graphene transparent conductor-based
touchscreen that is employed in (c) a commercial smartphone.
Graphene is a transparent and flexible conductor that holds great
promise for various material/device applications, including solar cells, light-emitting diodes (LED), touch panels, and smart windows or phones. Smartphone products with graphene touch screens are already on the market.
In 2013, Head announced their new range of graphene tennis racquets.
As of 2015, there is one product available for commercial use: a graphene-infused printer powder. Many other uses for graphene have been proposed or are under development, in areas including electronics, biological engineering, filtration, lightweight/strong composite materials, photovoltaics and energy storage.
Graphene is often produced as a powder and as a dispersion in a polymer
matrix. This dispersion is supposedly suitable for advanced composites,
paints and coatings, lubricants, oils and functional fluids, capacitors
and batteries, thermal management applications, display materials and
packaging, solar cells, inks and 3D-printers' materials, and barriers
and films.
In 2016, researchers have been able to make a graphene film that can absorb 95% of light incident on it.
Graphene is also getting cheaper. In 2015, scientists at the
University of Glasgow found a way to produce graphene at a cost that is
100 times less than the previous methods.
On August 2, 2016, BAC's new Mono model is said to be made out of graphene as a first of both a street-legal track car and a production car.
In January 2018, graphene based spiral inductors exploiting kinetic inductance at room temperature were first demonstrated at the University of California, Santa Barbara, led by Kaustav Banerjee. These inductors were predicted to allow significant miniaturization in radio-frequency integrated circuit applications.
The potential of epitaxial graphene on SiC for metrology has been
shown since 2010, displaying quantum Hall resistance quantization
accuracy of three parts per billion in monolayer epitaxial graphene.
Over the years precisions of parts-per-trillion in the Hall resistance
quantization and giant quantum Hall plateaus have been demonstrated.
Developments in encapsulation and doping of epitaxial graphene have led
to the commercialisation of epitaxial graphene quantum resistance
standards.
Toxicity
One review on graphene toxicity published in 2016 by Lalwani et al. summarizes the in vitro, in vivo, antimicrobial and environmental effects and highlights the various mechanisms of graphene toxicity. Another review published in 2016 by Ou et al. focussed on graphene-family nanomaterials (GFNs) and revealed several typical mechanisms such as physical destruction, oxidative stress, DNA damage, inflammatory response, apoptosis, autophagy, and necrosis.
A 2020 study showed that the toxicity of graphene is dependent on
several factors such as shape, size, purity, post-production processing
steps, oxidative state, functional groups, dispersion state, synthesis
methods, route and dose of administration, and exposure times.
In 2014 research at Stony Brook University showed that graphene nanoribbons,
graphene nanoplatelets and graphene nano–onions are non-toxic at
concentrations up to 50 μg/ml. These nanoparticles do not alter the
differentiation of human bone marrow stem cells towards osteoblasts
(bone) or adipocytes (fat) suggesting that at low doses graphene
nanoparticles are safe for biomedical applications.
In 2013 research at Brown University found that 10 μm few-layered
graphene flakes are able to pierce cell membranes in solution. They were
observed to enter initially via sharp and jagged points, allowing
graphene to be internalized in the cell. The physiological effects of
this remain uncertain, and this remains a relatively unexplored field.